JAK2 Assay Approval Supports Adherence to MPN Diagnostic Guidelines
,
Are there any ongoing efforts at Qiagen to advance the ipsogen JAK2 assay to detect some of the less common JAK2 mutations associated with myeloproliferative neoplasms?
The ipsogen JAK2 assay and workflow is designed and optimized toward the sensitive qualitative diagnostic testing of the JAK2 V617F mutation. Hence, an advancement towards other mutations is not reasonable. However, Qiagen is constantly looking to improve the molecular testing in MPNs and other leukemias.
About a year ago, Qiagen introduced the first ever IVD assay for Calreticulin (CALR) exon 9 mutations in the European Union. CALR exon 9 mutations comprise the second most frequent variants in ET and PMF patients and have been included as a major criterion in the 2016 revision to the WHO MPN guidelines.
Qiagen is also working on complete Sample to Insight Myeloid NGS panels for research applications on the GeneReader NGS system, which will then also include other less common JAK2 mutations such as the JAK2 exon 12 mutations mainly found in PV.
,
Last week, the US Food and Drug Administration (FDA) approved Qiagen’s ipsogen JAK2 V617F mutation assay for the use in diagnosing myeloproliferative neoplasms (MPNs). The FDA clearance allows for the diagnosis of both essential thrombocythemia (ET) and primary myelofibrosis (PMF).
Journal of Clinical Pathways spoke with Christoph Menzel, PhD, Director of Global Product Management MDx, about the implications of this approval and the importance of JAK2 testing to support treatment decision-making for MPNs.
-----
How may the ipsogen JAK2 assay impact the treatment landscape for ET, PMF, and other myeloproliferative neoplasms?
JAK2 V617F is the most frequent driver mutation in MPN and has had a World Health Organization (WHO) major criterion status for the diagnosis of polycythemia vera (PV), ET, and PMF for many years. The ipsogen JAK2 RGQ PCR kit now provides - for the first time - a FDA cleared corresponding diagnostic test, for which the robustness and clinical performance has been thoroughly established. This status and additional workflow features—such as the fully automated result interpretation—eases the implementation of guideline compliant JAK2 IVD testing in every lab. We believe this will support increased availability of high quality JAK2 testing and, as a consequence, treatments may benefit from this standardization of the diagnostic test.
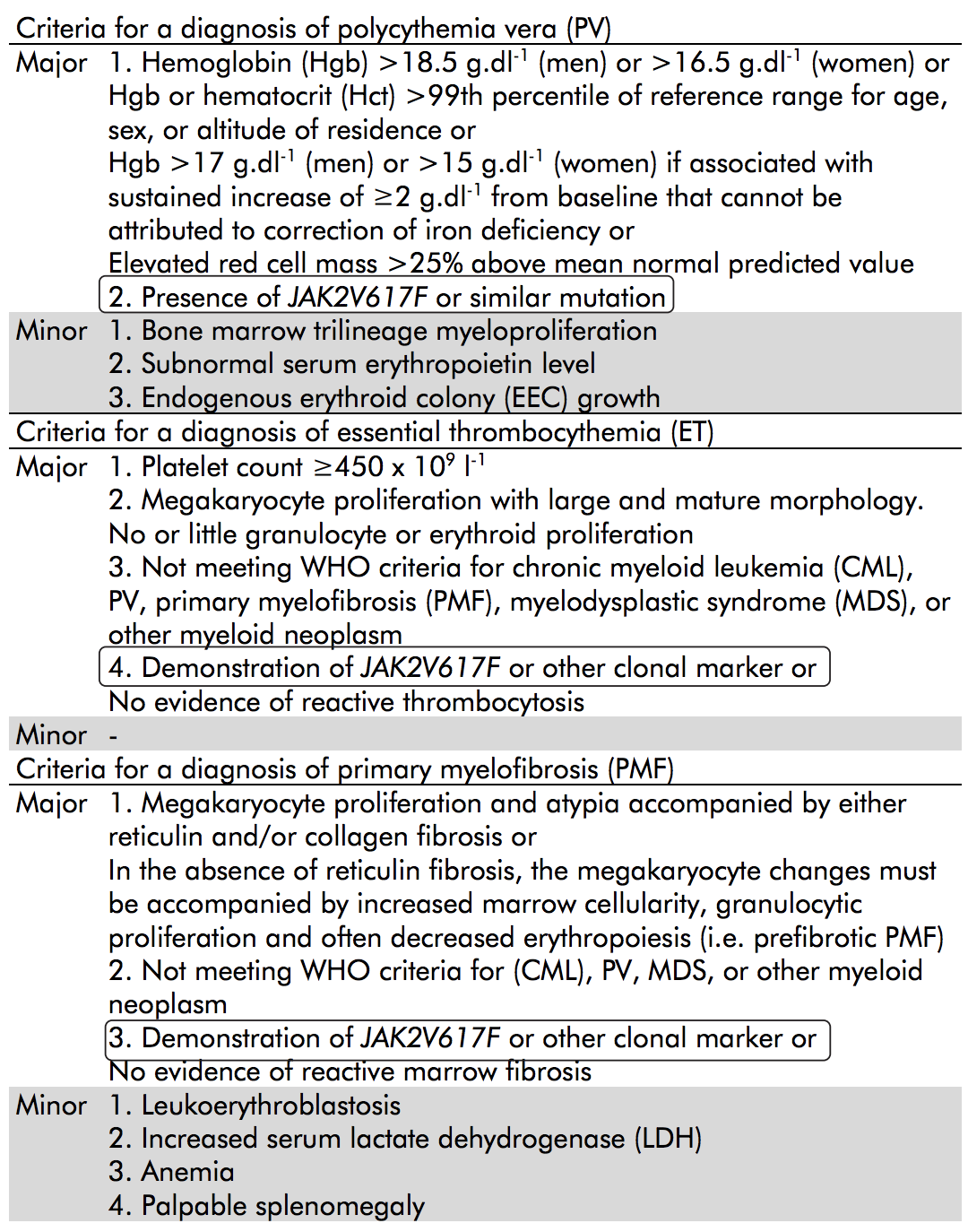
Table 1 details WHO criteria for the diagnosis of MPN, provided by Qiagen.
What effect may the assay have on improving adherence to diagnostic testing guidelines for patients at risk for MPNs?
The test not only supports the established WHO and other guidelines, it is also in line with published guidance concerning the diagnostic mutation allele frequency cut-off, uses the internationally recommended primer design sequences, and the overall workflow complies very well with the recommended and most widely used consecutive testing algorithm using JAK2 V617F as the first parameter. With just a single kit in a straight forward workflow, labs can now easily comply with various guidelines and recommendations.
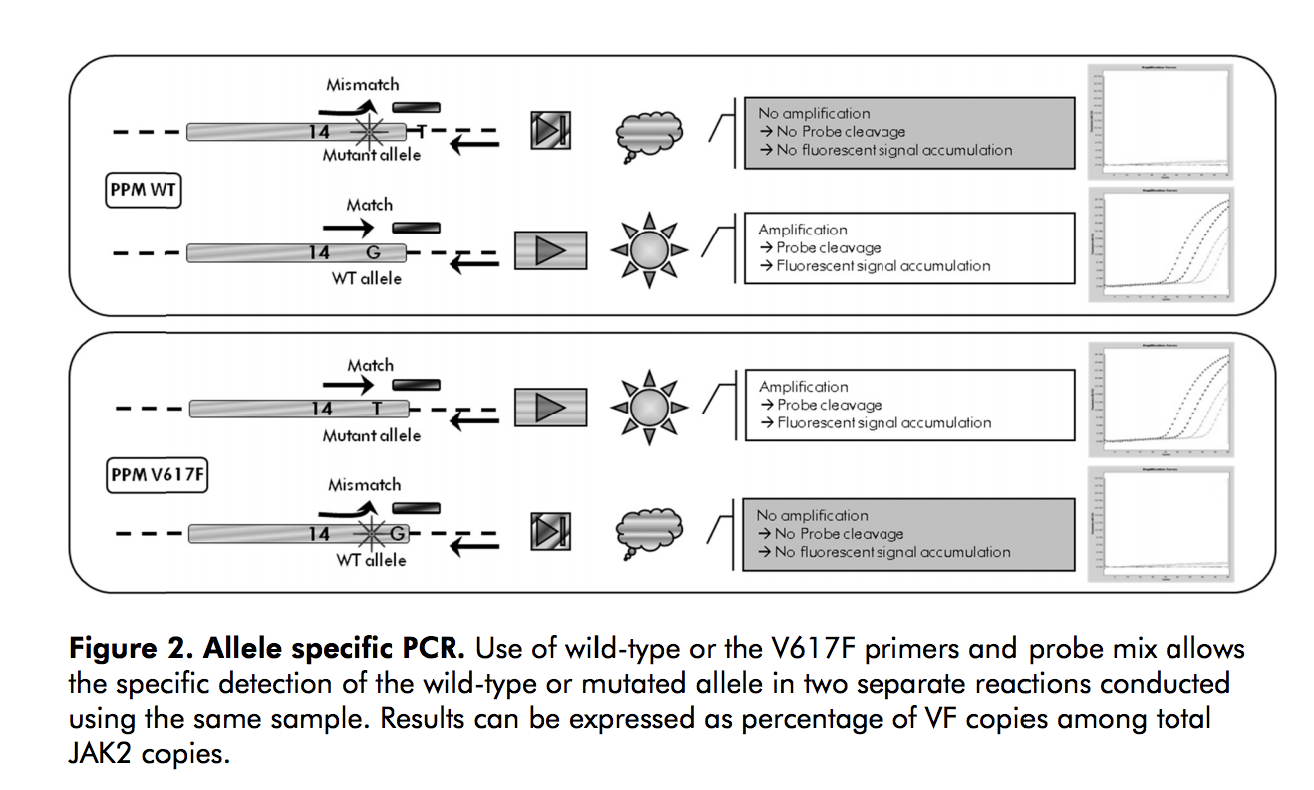
Figure 2 details the allele-specific polymerase chain reaction, provided by Qiagen.