Use of Continuous Topical Oxygen Therapy in Combination to Optimize the Chronic Wound Environment Prior to Cellular, Acellular, and Matrix-Like Product Application: A Retrospective Case Series
© 2024 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of ePlasty or HMP Global, their employees, and affiliates.
Abstract
Background. This retrospective case series details the use of a continuous topical oxygen therapy (cTOT) device for wound bed preparation prior to the application of cellular, acellular, and matrix-like products (CAMPs) on lower extremity wounds.
Methods. A retrospective records review was conducted in a single outpatient wound care center. Treatment consisted of 2 weeks of cTOT followed by CAMP application. Weekly wound photos and measurements were obtained through chart review. Patients included in this study did not achieve complete wound closure within the 2-week cTOT treatment period and were transitioned to application of a CAMP as per standard practice at the lead author's clinic.
Results. This study included 4 patients (5 wounds). The mean patient age was 71.8 years, and wound types included 3 diabetic foot ulcers (DFUs) and 2 venous leg ulcers (VLUs). The mean wound area reduction seen in this patient cohort was 74.7% and 76.1% at 4 and 6 weeks, respectively. Overall, a mean healing time of 8 weeks was noted across all wounds with a mean number of 6 CAMP applications.
Conclusions. Wound healing should be approached in an algorithmic manner, starting with wound bed optimization. In this patient cohort cTOT proved to be an effective way to improve the quality of the wound bed, in addition to the standard cleansing and debridement, prior to CAMP application. The authors believe that this combination of topical methods might have synergistic effects and improve wound healing, and the results of this study support this assumption.
Introduction
In chronic wounds, the physiological processes of wound healing are disrupted, resulting in delays in tissue repair and regeneration. Intricate and synchronized biological actions such as cellular proliferation, collagen deposition, angiogenesis, and tissue remodeling are integral to timely and efficient wound closure, which make chronic wound healing one of the most complex processes in the human body.1 Yet every refractory wound is unique, and clinicians must consider all the systemic and local factors that contribute to the varying pathophysiology in each patient. While the goals of wound management, including tissue repair support, infection prevention, pain reduction, devitalized tissue elimination, moist environment creation, and edema reduction, remain the same, understanding the effects of underlying comorbid conditions on the wound environment is of the utmost importance for successful wound management.2
Our current knowledge of useful wound healing therapies has largely been derived from studies comparing outcomes of various treatments with the standard of care on one specific wound type in cohorts not reflective of real-world patients.3 Additionally, the paucity of comparative effectiveness and head-to-head trials across a variety of wound types is a significant obstacle to the development of algorithmic or combination-therapy approaches to wound management. Therefore, consensus is lacking on optimal treatment strategies that can be used across the spectrum of care. Combination therapy, or the use of a variety of successive therapeutic approaches, may have the potential to tackle the complex challenges associated with delayed wound repair in individuals with hard-to-heal wounds of various etiologies.
Effective wound bed preparation is an essential part of any wound care protocol, aiding in the removal of barriers to healing and optimizing the wound environment for advanced interventions, such as cellular-based therapies, to bolster progression towards healing.4,5 In addition to routine cleansing and tissue debridement performed by wound professionals as part of wound hygiene practices, evidence-based adjunctive treatments such as negative pressure wound therapy (NPWT) or continuous topical oxygen therapy (cTOT) can be used to support good wound bed preparation as the standard of care.4,6-8
Prolonged tissue hypoxia as a result of poor vasculature function, endothelial tissue loss, or edema can lead to multiple tissue complications such as infection, delayed healing, and wound dehiscence and is a major risk for skin graft failure.9-11 Large defects may provide challenges regarding nutrient and oxygen diffusion and, as a result, nutrient-deprived cells that are distant from the surrounding capillaries may experience impaired proliferation and migration.12 Oxygen plays an essential role in multiple wound-healing processes including oxidative killing of bacteria, cellular signaling and proliferation, collagen deposition, and angiogenesis, which are all essential to the preparation of the wound environment for receiving grafts or tissue products.8 It is also important for oxygen to reach tissue products to maximize their potential benefits to the wound site.13 Supplemental oxygen has been shown to stimulate epidermal reconstruction in human skin equivalents, leading to enhanced migration and accelerated epidermal maturation.14 cTOT represents a significant advancement in delivering supplemental oxygen to chronic wound tissues. Additionally, cTOT treatment is easy to use in a wide range of care settings and a variety of chronic wound types.7,8,15
Cellular, acellular, and matrix-like products (CAMPs), previously referenced as cellular tissue products (CTPs), are "a broad category of biomaterials, synthetic materials, or biosynthetic matrices that support repair or regeneration of injured tissues through various mechanisms of action."16 There is a large body of evidence published in the literature on the use of CAMPs in the treatment of chronic wounds. However, the quality and quantity of evidence differs from product to product.16 Additionally, there is scant data in the literature to assist healthcare professionals in decision-making regarding the best product to use for a particular indication.16
A recent consensus panel reporting on the optimized use of CAMPs outlined several essential steps that should be performed prior to CAMP application, with adequate wound bed preparation being among these recommendations.16 This ideology aligns with the Centers for Medicare and Medicaid Services (CMS) Local Coverage Determination (LCD), which outlines the covered indications for CAMP application. The LCD clearly dictates that, prior to CAMPs application, the implementation of a treatment plan to produce a clean granular wound base without evidence of infection is a requirement.17
The following retrospective case series details an algorithmic approach to the use of a cTOT device (NATROX O2, Inotec AMD Ltd) for wound bed preparation to optimize the wound environment prior to the application of CAMPs in an outpatient wound clinic on both diabetic foot ulcers (DFUs) and venous leg ulcers (VLUs). The objective of this study was to determine the feasibility of a larger investigation into the serial use of cTOT and CAMPs in the management of hard-to-heal wounds of the lower extremity.
Materials and Methods
This single-center, retrospective case review was conducted to examine the outcomes of a chronic wound therapy algorithm consisting of 2 weeks of wound bed optimization with cTOT followed by CAMP application.
The retrospective case study was conducted in accordance with the Health Insurance Portability and Accountability Act guidelines and adhered to the tenets of the International Conference on Harmonization E6 Good Clinical Practice (ICH GCP) and the Declaration of Helsinki. This retrospective review was exempt from IRB approval. All patients provided written informed consent to publish the case details and the associated de-identified image assessments. No compensation was provided for participation.
All wounds were considered nonhealing prior to the use of cTOT because they had failed to achieve a wound area reduction of at least 50% after the standard-of-care treatment for at least 4 weeks. Previous wound management varied and included debridement, offloading, compression bandages, alginates, collagen, gelling hydrofibers, foam dressings, and topical antimicrobial agents.
Wound assessment and treatment
Wound measurements captured via an automated 3-dimensional wound-imaging device were recorded for each patient visit. The cTOT device (NATROX O2, NATROX Wound Care; Figure 1) was applied to the wound per the manufacturer's instructions for use. Patients were seen at least once weekly in the clinic for 2 consecutive weeks. Weekly wound photos, measurements, and tissue oxygenation measured using near-infrared spectroscopy were obtained through chart review. All wounds had regular debridement to remove devitalized tissue per the clinician's discretion. Wounds were bandaged with a variety of inert semiocclusive dressing materials to manage exudate and promote a moist wound-healing environment. In all cases, plantar foot wounds were offloaded via total contact cast or removable walking boot, and all venous ulcers were treated with compression bandaging as part of the standard of care. Patients included in this study did not achieve complete wound closure within the 2-week cTOT treatment period and were transitioned to the application of a CAMP as per standard practice at the lead author's clinic.

Figure 1. Image of the cTOT device (NATROX O2, NATROX Wound Care).
A variety of CAMP products including amniotic tissue grafts and acellular dermal matrices were used based on the lead author's current selection method, which considers clinical wound type, wound assessment, goals of healing, and product mechanism of action. All CAMPs were applied per the manufacturer's guidelines. No products were used off-label. Reapplication of the CAMP was also performed at weekly intervals in all patients included in this series. The CAMP was covered with a non-adherent dressing followed by an inert moisture-managing secondary dressing and appropriate offloading or compression dressings if applicable. All patients were followed in the lead author's wound clinic until complete wound closure was achieved.
Results
Four patients with a total of 5 wounds were included in this case review. Patient demographics are shown in Table 1. The mean patient age was 71.8 years and wound types included 3 diabetic foot ulcers (DFUs) and 2 venous leg ulcers (VLUs). All patients had an ankle-brachial index of at least 0.9 mm Hg but not greater than 1.3 mm Hg. Additionally, all wounds were negative for clinical signs and symptoms of infection prior to beginning cTOT.

Table 1. Patient Demographics and Baseline Wound Information
The mean wound area reduction seen in this patient cohort undergoing therapy with cTOT and subsequent CAMPs was 74.7% and 76.1% at 4 and 6 weeks, respectively, as shown in Figure 2. Patient 1 showed an increased wound size at week 6 compared with week 4; however, the wound did progress rapidly to healing by week 12. Overall, improvement in the wound bed tissue characteristics was observed following cTOT treatment compared with baseline.

Figure 2. The mean wound area reduction for patients undergoing therapy with cTOT and subsequent CAMPs.
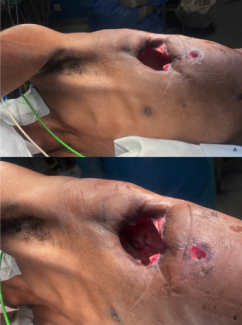
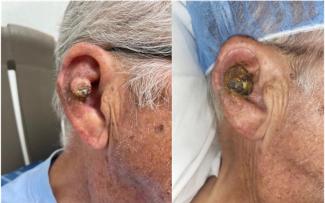
Overall, a mean healing time of 8 weeks was noted across all wounds, with a mean number of 6 CAMP applications used per patient through to wound closure (Table 2). Of the 5 wounds, 1 wound healed by week 4 and another 2 healed by week 6. The remaining 2 wounds completed wound resolution by week 12 (Table 2, Figure 2). Specific case examples are illustrated in Figure 3. No adverse events were reported throughout.

Table 2. Time to Healing and Number of Camp Applications Used Per Patient

Figure 3. Examples from 2 cases included in the study.
Discussion
Chronic wound healing is a complex and intricate process requiring the activation of multiple overlapping steps and cellular functions.18 To develop the best treatment plan, clinicians must consider therapies that address a variety of patient-specific causative factors that contribute to wound chronicity such as patient age, underlying comorbid conditions, infection status of the wound, tissue perfusion, and tissue quality, just to name a few. Thus, standard-of-care wound-treatment algorithms include the removal of necrotic tissue, the selection of dressings that maintain a moist wound environment, the control of bacterial contamination or treatment of wound infections, and the reestablishment of blood flow to the wounded tissues.5,19 Despite the employment of these foundational interventions, many wounds will remain unhealed. When wounds fail to enter a healing trajectory, it is recommended to leverage advanced wound care therapies.8
Modern advances in the scientific study of wound healing and the identification of the molecular pathophysiology of hard-to-heal wounds have yet to translate into widely used multimodal wound care algorithms. While new commercially available products continue to enter the market, there remains a need to develop structured multimodal wound care treatment plans to optimize individual patient outcomes. Identifying wound care therapies capable of working serially or in tandem to overcome the nebulous tissue microenvironment found within hard-to-heal wounds will enable clinicians to practice precision medicine and develop patient-centric therapeutic regimens.
Skin is the largest organ system of the body. It is the first line of defense during birth, and it remains the last line of defense during life. Adequate oxygenation is required for basic skin tissue perfusion and throughout the wound healing cascade. cTOT has been established as a beneficial therapy that supports wound management through both meta-analyses and randomized controlled trials evidence.20-27 International expert guidance and consensus documents have advocated for cTOT to be included in hard-to-heal wound care regimens based on the mounting level of published evidence.8,28-32 cTOT supports wound bed optimization through a variety of mechanisms. Reversing the hypoxic conditions of the chronic wound environment through the addition of cTOT helps to bolster the immune system function. Oxygen plays a key role in supporting immune cell migration and activity, leading to an upregulation of phagocytosis and bacterial killing via reactive oxygen species generation.33 In a study by Hunter et al, the authors concluded that topical oxygen therapy, when applied to the wound bed, modified the tissue microbiome to closely resemble that of intact skin, thus contributing to wound healing through suppression of chronic inflammation.34 Furthermore, changing the oxygen gradient in the wound bed via cTOT helps recruit fibroblast and epithelial cells to support granulation tissue formation and wound repair and regeneration.35 All of these clinical benefits provide an optimized wound environment to enhance the success of CAMPs and, as observed in this small case series, this sequential approach can aid healing. This may be further maximized by longer durations of cTOT treatment.
Additionally, CAMPs play an important role in wound management. When used appropriately, these products can reestablish a favorable wound environment that expedites wound healing. While the mechanism of action varies from product to product, many CAMPs work by bolstering cellular functions or activating the molecules that are intrinsic to wound closure.36
In a recently published CAMPs consensus document, the authors stressed the importance of wound bed preparation as a critical step for successful CAMPs use.16 Furthermore, the consensus concluded that inflammation and infection should ideally be resolved prior to CAMP application.16 The publication cautions that lack of adequate optimization of the wound bed will ultimately lead to CAMP failure.16 The benefits of adequate oxygenation of a wound when using tissue substitutes have also been demonstrated previously in vitro, with early keratinocyte migration, enhanced collagen deposition, and accelerated epidermal maturation noted in oxygen-supplemented tissue compared with controls.14
While the exact mode of action varies from product to product, the role of CAMPs in wound management is to reinforce the wound's innate healing mechanisms through supporting intra- and extra-cellular communication and activities.16 This dynamic reciprocity supports the reestablishment of the wound healing processes and helps restore normal tissue repair functions.16 While CAMPs can stimulate the extracellular matrix, these products will have limited long-term success without oxygen.14 Therefore, initiating topical oxygen early for wound bed optimization is very important and should be considered in the wound healing algorithm, as recently highlighted in the M.O.I.S.T wound care guidance document.4
It should be noted that stimulation of cellular processes in stagnant wounds can lead to increased exudate and autolytic debridement of necrotic tissue, as noted in previous studies using cTOT.21 This may explain the initial increase in wound size that was observed in patient 1. Nevertheless, this patient went on to fully heal by week 12, highlighting the benefits of this sequential treatment.
The mean time to complete closure for the 5 wounds reported in this study was 8 weeks, with a mean of 6 applications of CAMPs. While different CAMPs were used in this study, possibly influencing the number of applications, this data highlights an interesting observation that should be explored further in comparative studies to resolutely ascertain if the synergy of sequential cTOT and CAMPs use may reduce the number of applications required with a corresponding decrease in time to healing.
Limitations
The limitation of this project is that it is a single-site, retrospective study on a small sample size of subjects, making it difficult to draw sound conclusions about the effects of cTOT and CAMPs used sequentially. However, the results of this retrospective case series support the feasibility of future studies to include a larger patient cohort to determine the optimal treatment pathways for patients.
Conclusions
This small feasibility study is a step toward transforming wound care into a precision practice. Wound healing should be approached in a systematic and algorithmic way, starting with wound bed optimization. In this patient cohort, cTOT proved to be an effective way to improve the quality of the wound bed, in addition to the standard cleansing and debridement, prior to CAMP application. The authors believe that this combination of topical methods might have synergistic effects and improve wound healing, and the results of this study support this assumption. With looming limitations in the number of CAMP applications permitted under various LCD/Local Coverage Article policies, as well as the constraints in accessing certain CAMPs, finding innovative methods to improve wound healing will have great value across all clinical settings.
Acknowledgments
Authors: Naz Wahab, MD1; Windy Cole, DPM2; Emma Woodmansey, PhD3
Affiliations: 1Wound Care Experts, Las Vegas, Nevada; 2Kent State University College of Podiatric Medicine, Independence, Ohio; 3NATROX Wound Care, Cambridge, United Kingdom.
Correspondence: Windy Cole, DPM; wcole4@kent.edu
Ethics: The retrospective case study was conducted in accordance with Health Insurance Portability and Accountability Act guidelines, adhered to tenets of the International Conference on Harmonization E6 Good Clinical Practice (ICH GCP) and the Declaration of Helsinki. This retrospective review was exempt from IRB approval. All patients provided written informed consent to publish the case details and associated de-identified image assessments. No compensation was provided for participation.
Disclosures: Dr Cole is a member of the NATROX Wound Care Clinical Advisory Board. Dr Woodmansey is the NATROX Wound Care Global Clinical Director. The remaining author discloses no financial or other conflicts of interest.
References
1. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99(1):665-706. doi:10.1152/physrev.00067.2017
2. Beyene RT, Derryberry SL Jr, Barbul A. The effect of comorbidities on wound healing. Surg Clin North Am. 2020;100(4):695-705. doi:10.1016/j.suc.2020.05.002
3. Carter MJ, Fife CE, Walker D, Thomson B. Estimating the applicability of wound care randomized controlled trials to general wound-care populations by estimating the percentage of individuals excluded from a typical wound-care population in such trials. Adv Skin Wound Care. 2009;22(7):316-24. doi:10.1097/01.ASW.0000305486.06358.e0
4. Dissemond J, Chadwick P, Weir D, et al. M.O.I.S.T. concept for the local therapy of chronic wounds: an international update. Int J Low Extrem Wounds. 2024:15347346241245159. doi:10.1177/15347346241245159
5. Schultz GS, Barillo DJ, Mozingo DW, Chin GA; Wound Bed Advisory Board Members. Wound bed preparation and a brief history of TIME. Int Wound J. 2004;1(1):19-32. doi:10.1111/j.1742-481x.2004.00008.x
6. Fletcher J, Dark J, Dhoonmoon L, et al. Best practice statement. Active Treatment of non-healing wounds in the community. Wounds UK. 2022. https://wounds-uk.com/wp-content/uploads/2023/02/040b5d3ac0786d909eb7141cb47bbdf5.pdf. Accessed May 30, 2024.
7. Serena T, Andersen C, Cole W, Garoufalis M, Frykberg R. Guidelines for the use of topical oxygen therapy in the treatment of hard-to-heal wounds based on a Delphi consensus. J Wound Care. 2021;30(Sup9):S30-S34. doi:10.12968/jowc.2021.30.Sup9.S30
8. Frykberg R, Andersen C, Chadwick P, et al. Use of topical oxygen therapy in wound healing. J Wound Care. 2023;32(Sup8b):S1-S32. doi:10.12968/jowc.2023.32.Sup8b.S1
9. Leaper D. Perfusion, oxygenation and warming. Int Wound J. 2007;4 Suppl 3(Suppl 3):4-8. doi:10.1111/j.1742-481X.2007.00382.x
10. Hopf HW, Hunt TK, West JM, et al. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg. 1997;132(9):997-1004; discussion 1005. doi:10.1001/archsurg.1997.01430330063010
11. Turissini JD, Elmarsafi T, Evans KK, Kim PJ. Major risk factors contributing to split thickness skin graft failure. Georgetown Medical Review. 2019;3(1). doi:10.52504/001c.7755
12. Vecin NM, Kirsner RS. Skin substitutes as treatment for chronic wounds: current and future directions. Front Med (Lausanne). 2023;10:1154567. doi:10.3389/fmed.2023.1154567
13. Woodroof A, Moote J, Polansky J, Haith LR Jr, Hickerson WL. Preliminary study of wound oxygenation comparing skin substitutes and dressings. Eplasty. 2023;23:e52.
14. Kairuz E, Upton Z, Dawson RA, Malda J. Hyperbaric oxygen stimulates epidermal reconstruction in human skin equivalents. Wound Repair Regen. 2007;15(2):266-274. doi:10.1111/j.1524-475X.2007.00215.x
15. Cullen B, Al Eisa AA, Kaufman H, et al. Consensus round table meeting: portable oxygen therapy for healing complex wounds. Wounds International. 2018. https://woundsinternational.com/wp-content/uploads/2023/02/92c7b47255eee2a1511106a60516e7ed.pdf
16. Wu S, Carter M, Cole W, et al. Best practice for wound repair and regeneration use of cellular, acellular and matrix-like products (CAMPs). J Wound Care. 2023;32(Sup4b):S1-S31. doi:10.12968/jowc.2023.32.Sup4b.S1
17. Centers for Medicare and Medicaid Services. Application of skin substitute grafts for treatment of DFU and VLU of lower extremities. L36377. January 8, 2019. Updated November 21, 2019. https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdId=36377&ver=7. Accessed April 15, 2024.
18. Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599-610. doi:10.1007/s12325-017-0478-y
19. Atkin L, Bućko Z, Conde Montero E, et al. Implementing TIMERS: the race against hard-to-heal wounds. J Wound Care. 2019;23(Sup3a):S1-S50. doi:10.12968/jowc.2019.28.Sup3a.S1
20. Serena TE, Bullock NM, Cole W, et al. Topical oxygen therapy in the treatment of diabetic foot ulcers: a multicentre, open, randomised controlled clinical trial. J Wound Care. 2021;30(Sup5):S7-S14. doi:10.12968/jowc.2021.30.Sup5.S7
21. Yu J, Lu S, McLaren AM, Perry JA, Cross KM. Topical oxygen therapy results in complete wound healing in diabetic foot ulcers. Wound Repair Regen. 2016;24(6):1066-1072. doi:10.1111/wrr.12490
22. Al-Jalodi O, Kupcella M, Breisinger K, Serena TE. A multicenter clinical trial evaluating the durability of diabetic foot ulcer healing in ulcers treated with topical oxygen and standard of care versus standard of care alone 1 year post healing. Int Wound J. 2022;19(7):1838-1842. doi:10.1111/iwj.13789
23. Thanigaimani S, Singh T, Golledge J. Topical oxygen therapy for diabetes-related foot ulcers: a systematic review and meta-analysis. Diabet Med. 2021;38(8):e14585. doi:10.1111/dme.14585
24. Connaghan F, Avsar P, Patton D, O'Connor T, Moore Z. Impact of topical oxygen therapy on diabetic foot ulcer healing rates: a systematic review. J Wound Care. 2021;30(10):823-829. doi:10.12968/jowc.2021.30.10.82
25. Sethi A, Khambhayta Y, Vas P. Topical oxygen therapy for healing diabetic foot ulcers: a systematic review and meta-analysis of randomised control trials. Health Sciences Review. 2022;3:100028. doi:10.1016/j.hsr.2022.100028
26. Sun XK, Li R, Yang XL, Yuan L. Efficacy and safety of topical oxygen therapy for diabetic foot ulcers: an updated systematic review and meta-analysis. Int Wound J. 2022;19(8):2200-2209. doi:10.1111/iwj.13830
27. Carter MJ, Frykberg RG, Oropallo A, et al. Efficacy of topical wound oxygen therapy in healing chronic diabetic foot ulcers: systematic review and meta-analysis. Adv Wound Care (New Rochelle). 2023;12(4):177-186. doi:10.1089/wound.2022.0041
28. Chen P, Vilorio NC, Dhatariya K, et al. Guidelines on interventions to enhance healing of foot ulcers in people with diabetes (IWGDF 2023 update). Diabetes Metab Res Rev. 2024;40(3):e3644. doi:10.1002/dmrr.3644
29. American Diabetes Association Professional Practice Committee. 12. Retinopathy, neuropathy, and foot care: standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S231-S243. doi:10.2337/dc24-S012.
30. ElSayed NA, Aleppo G, Aroda VR, et al; American Diabetes Association. 12. Retinopathy, neuropathy, and foot care: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S203-S215. doi:10.2337/dc23-S012
31. Lavery LA, Suludere MA, Attinger CE, et al. WHS (Wound Healing Society) guidelines update: diabetic foot ulcer treatment guidelines. Wound Repair Regen. 2024;32(1):34-46. doi:10.1111/wrr.13133
32. Pacheco YJ, Marin ELN, Ocampo DB, et al. Consenso de expertos sobre la eficacia clínica y directrices sobre la terapia de oxígeno transdérmico continuo para la cicatrización de las heridas complejas o difíciles de cicatrizar. J Wound Care. 2023;32(LatAm sup 10):1-37. doi:10.12968/jowc.2023.32.LatAm_sup_10.1
33. Sen CK. Wound healing essentials: let there be oxygen. Wound Repair Regen. 2009;17(1):1-18. doi:10.1111/j.1524-475X.2008.00436.x
34. Hunter P, Greco E, Cross K, Perry J. Topical oxygen therapy shifts microbiome dynamics in chronic diabetic foot ulcers. Wounds. 2020;32(3):81-85.
35. Asmis R, Qiao M, Zhao Q. Low flow oxygenation of full-excisional skin wounds on diabetic mice improves wound healing by accelerating wound closure and reepithelialization. Int Wound J. 2010;7(5):349-357. doi:10.1111/j.1742-481X.2010.00716.x
36. Urciuolo F, Casale C, Imparato G, Netti PA. Bioengineered skin substitutes: the role of extracellular matrix and vascularization in the healing of deep wounds. J Clin Med. 2019;8(12):2083. doi:10.3390/jcm8122083