Role of the Plastic Surgeon in Tuberculosis Treatment
© 2023 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of ePlasty or HMP Global, their employees, and affiliates.
Abstract
Background. Tuberculous empyema is rare. Its treatment requires oral antituberculous drugs, empyema drainage, and in severe cases, decortication and pneumectomy. In the presence of tuberculosis, lung resection has a high risk of postoperative bronchopleural fistula (BPF) and empyema. Treatment includes drainage, fistula occlusion, dead space obliteration, and infection control. Muscle flap transfer allows BPF occlusion and dead space obliteration.
Methods. This report presents a case of a 63-year-old man with tuberculosis and postoperative BPF with empyema after pleural decortication and left lower lobe resection. The empyema was drained, and antituberculous drugs were started. The BPF was occluded with a latissimus dorsi and serratus anterior chimeric muscle flap, and the remaining thoracic dead space and chest wall defect were reconstructed with a pedicled pectoralis major myocutaneous flap.
Results. Healing occurred uneventfully, and the patient was discharged from the hospital after 2 weeks.
Conclusions. This type of thoracic defect is rare nowadays, especially in the setting of tuberculous infections. Although workhorse flaps like latissimus dorsi or pectoralis major flaps have been progressively surpassed by more elegant solutions like fasciocutaneous pedicled flaps and free flaps, they must still be considerations in the decision-making process of a reconstructive surgeon, and flap choice must be made on a case-by-case basis.
Introduction
Although the incidence of tuberculosis (TB) has decreased significantly worldwide in the last decades, it is still a public health issue, namely in high TB burden areas like the African countries. According to the World Health Organization Global Tuberculosis Report 2021, TB affects 220 per 100,000 persons in African countries compared with 25 per 100,000 persons in Europe.1
Tuberculous empyema is a rare complication of pleuropulmonary TB.2,3 Its treatment requires oral antituberculous drugs, empyema drainage, and in severe cases, decortication and pneumectomy.2,3 In the presence of an infectious condition like TB, lung resection has a particularly high risk of postoperative bronchopleural fistula (BPF) and empyema.4
BPF is an uncommon complication of thoracic surgeries and is usually associated with empyema.4-7 It is a severe condition associated with significant morbidity and mortality, and it must be promptly treated when diagnosed.5-8 BPF with empyema treatment is challenging and includes effective drainage, fistula occlusion, dead space obliteration, and infection control.5,7
Regional or free muscle flap transfer allows BPF occlusion and dead space obliteration, being a well-established technique for intrathoracic defect coverage.5,7,9



Methods
We present a case of a 63-year-old man from Cape Verde who was transferred to our hospital due to postoperative empyema after pleural decortication and left lower lobe resection. Bronchoalveolar lavage analysis was positive for TB, and oral antituberculous drugs were started. An open-window thoracostomy (OWT) with partial resection of the sixth and seventh ribs was performed to drain the empyema, and a BPF was detected. The wound was cleaned with antiseptic-soaked dressings on a daily basis for 1 month.
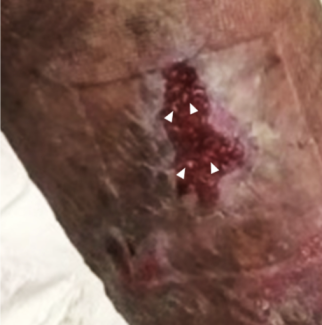
Once the wound bed was prepared and infection controlled (Figure 1), the BPF was occluded with a latissimus dorsi and serratus anterior chimeric muscle flap (Figure 2). The decision to use a bulkier chimeric flap was based on the large size of the defect and on the presence of a posterolateral thoracotomy scar over the lower part of the latissimus dorsi (Figure 3). The flap was moved to the desired position through the OWT opening after removal of a segment of the fifth rib superiorly to facilitate its inset. Direct BPF stump closure was not possible, and the flap was sutured to it in an airtight manner. The peripheral areas of the muscles were used to partially obliterate the left lower lobe corresponding space, but a significant residual defect was still present. In the postoperative period, daily antiseptic-soaked dressing changes were continued and the wound was regularly inspected to ensure flap viability and to confirm a stable BPF closure.


Results
After 1 month, a second reconstructive surgery was performed to address the remaining thoracic dead space and the chest wall defect; a pedicled pectoralis major myocutaneous flap was transferred laterally (Figure 4). A suction drain was placed under the flap, and the donor site was covered with a split-thickness skin graft. Healing occurred uneventfully, and the patient was discharged from the hospital 2 weeks after the second surgery, maintaining the oral antituberculous drugs (Figure 5).
Discussion
Tuberculous empyema is a rare complication of pleuropulmonary TB.2,7,10 It is a chronic active infection of the pleura with purulent pleural effusion and is commonly accompanied by pleural thickening and calcifications that can progress to fibrothorax.10 Treatment must include oral antituberculous drugs and empyema drainage; decortication is usually also necessary to allow lung re-expansion, and sometimes pneumectomy is indicated to eliminate all the infected tissue.3
BPF is a communication between the bronchial tree and the pleural space.5,6 It is an uncommon but severe complication after pulmonary resections and is commonly associated with empyema.5-7
Treatment of BPF is challenging and involves effective drainage, fistula occlusion, dead space obliteration, and infection control.5,7 Open-window thoracostomy is effective in draining the empyema and facilitates BPF identification.5 Occlusion of the fistula can be achieved by thoracoplasty or by muscle flap transfer.5 Thoracoplasty is an effective but mutilating procedure that is rarely performed nowadays.5 Muscle flap transfer is a well-established technique for reconstruction of intrathoracic defects; it is effective in BPF occlusion and dead space obliteration and is also helpful in controlling infection.5,7,9
The most commonly used pedicled muscle flaps are latissimus dorsi, serratus anterior, pectoralis major, pectoralis minor, rectus abdominis and intercostal muscles.5-8,11 Latissimus dorsi muscle flap is the most widely used strategy since it is the largest and most reliable and has low associated morbidity.5-7 It can be combined with serratus anterior muscle to form a chimeric flap if a bulkier flap is necessary.5,11 Pectoralis major flap is another workhorse flap often used for intrathoracic defects coverage given its proximity, reliability, and low functional deficit.9 If pedicled muscle flaps are not an option, alternatives include omental and free muscle flaps.6,8,9,11
Our case was particularly challenging: in addition to the patient being very thin and having a large postlobectomy defect, he had a history of posterolateral thoracotomy with distal division of the latissimus dorsi muscle, potentially reducing the size of the flap. Therefore, we opted to perform a chimeric muscle flap composed of latissimus dorsi and serratus anterior muscles. The flap allowed successful BPF occlusion and partial dead space obliteration. However, a significant part of the postlobectomy defect persisted.
Once BPF occlusion was stable and the wound adequately cleaned, we decided to fill the remaining defect with a pedicled myocutaneous pectoralis major flap. This flap was transferred laterally and allowed partial intrathoracic filling and lateral chest wall coverage.
The choice of the second flap was difficult. Locorregional options like the pectoralis major or rectus abdominis myocutaneous flaps were available, but their size and arc of rotation were limited by the patient’s body habitus. On the other hand, an omental flap was particularly risky given the history of pleural tuberculous infection and the possibility of intraperitoneal spread of the infection. Additionally, free flaps were hindered by the paucity of recipient vessels around the defect. Therefore, keeping in mind the necessity of providing good quality tissues while reducing the risk of postoperative complications that would impair wound healing and infection resolution, we opted for a pedicled pectoralis major myocutaneous flap. Our choice proved to be effective and allowed an uneventful wound healing and patient recovery, enabling hospital discharge after 2 weeks following the reconstruction.
Conclusions
This type of thoracic defect is rare, especially in the setting of tuberculous infections. However, the rationale for reconstruction follows the same principles as in other defects. Plastic surgeons should have a broad armamentarium of reconstructive solutions and adapt them not only to the defect but to the patient as a whole. Although workhorse flaps like latissimus dorsi or pectoralis major flaps have been progressively surpassed by more elegant solutions like fasciocutaneous pedicled flaps and free flaps, they must still be a consideration in the decision-making process of a reconstructive surgeon.
Acknowledgments
Afilliations: 1Department of Plastic, Reconstructive and Aesthetic Surgery, and Burn Unit, Centro Hospitalar Universitário de São João, Porto, Portugal; 2Department of Surgery and Physiology, Faculty of Medicine, University of Porto, Porto, Portugal; 3Department of Thoracic Surgery, Centro Hospitalar Universitário de São João, Porto, Portugal
Correspondence: Margarida Sofia Soares Mendes, MD; margarida_mendes900@hotmail.com
Disclosures: The authors disclose no financial or other conflicts of interest.
References
1. World Health Organization. Global Tuberculosis Report. Published October 14, 2021. Accessed October 2, 2023. http://iris.who.int/bitstream/handle/10665/346387/9789240037021-eng.pdf
2. Long R, Barrie J, Stewart K, Peloquin CA. Treatment of a tuberculous empyema with simultaneous oral and intrapleural antituberculosis drugs. Can Respir J. 2008;15(5):241-243. doi:10.1155/2008/747206
3. Long R, Barrie J, Peloquin CA. Therapeutic drug monitoring and the conservative management of chronic tuberculous empyema: case report and review of the literature. BMC Infect Dis. 2015;15:327. doi:10.1186/s12879-015-1093-7
4. Abolhoda A, Bui TD, Milliken JC, Wirth GA. Pedicled latissimus dorsi muscle flap: routine use in high-risk thoracic surgery. Tex Heart Inst J. 2009;36(4):298-302.
5. He Z, Shen L, Xu W, He X. Effective treatment of bronchopleural fistula with empyema by pedicled latissimus dorsi muscle flap transfer: two case report. Medicine (Baltimore). 2020;99(41):e22485. doi:10.1097/MD.0000000000022485
6. Lu C, Feng Z, Ge D, et al. Pedicle muscle flap transposition for chronic empyema with persistent bronchopleural fistula: experience of a single clinical center in China. Surg Today. 2016;46(10):1132-1137. doi:10.1007/s00595-015-1288-y
7. Pairolero PC, Arnold PG, Trastek VF, Meland NB, Kay PP. Postpneumonectomy empyema. The role of intrathoracic muscle transposition. J Thorac Cardiovasc Surg. 1990;99(6), 958-968.
8. Zanotti G, Mitchell JD. Bronchopleural fistula and empyema after anatomic lung resection. Thorac Surg Clin. 2015;25(4):421-427. doi:10.1016/j.thorsurg.2015.07.006
9. Fricke A, Bannasch H, Klein HF, et al. Pedicled and free flaps for intrathoracic fistula management. Eur J Cardiothorac Surg. 2017;52(6):1211-1217. doi:10.1093/ejcts/ezx216
10. Shaw JA, Diacon AH, Koegelenberg C. Tuberculous pleural effusion. Respirology. 2019;24(10):962-971. doi:10.1111/resp.13673
11. Watanabe K, Kiyokawa K, Ino K, Nishi Y, Rikimaru H, Inoue Y. Treatment strategies for refractory pulmonary fistulae using a latissimus dorsi muscle flap. J Plast Reconstr Aesthet Surg. 2011;64(8):1014-1021. doi:10.1016/j.bjps.2011.02.007