Lower Extremity Microsurgery in a Patient With Combined Sickle Cell Disease and Hemophilia: A Case Report and Literature Review
© 2023 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of ePlasty or HMP Global, their employees, and affiliates.
Abstract
The combination of hemophilia and sickle cell disease poses unique challenges in the perioperative management of patients requiring free tissue transfer (FTT). Extremity FTT requires mitigation of risk factors related to perioperative bleeding and microvascular thrombosis. This case report highlights the nuances of managing an open ankle fracture in a patient with both sickle cell trait and severe hemophilia A. The narrative and discussion highlights the importance of a multidisciplinary team with regard to executing a limb salvage plan in the setting of complex medical decision-making.
Introduction
Free tissue transfer (FTT) was first performed in 1959 for esophageal reconstruction and has since revolutionized the management of many traumatic, oncologic, and congenital defects.1 While FTT is a safe and evidence-based surgery, it carries significant risks, including respiratory failure, pulmonary emboli, septic shock, and myocardial infarctions.1 The decision to perform FTT must evaluate risk factors in order to identify the best candidates. Certain conditions, particularly bleeding disorders, are important considerations.
Hemophilia is a rare yet severe bleeding disorder that is estimated to affect 400,000 people worldwide.2 Severity of disease in hemophilia A is directly correlated to factor VIII levels.2 Inadequate medical management of patients with hemophilia A who require FTT can result in hematomas, necrosis of the flap, and poor wound healing.3 In the setting of microvascular surgery, concerns around bleeding at the flap site must be balanced with a hypercoagulable state as a result of overcorrection.4 There are few cases discussing the management of patients with hemophilia A and B who require flap surgery.2-4 Management of bleeding complications focuses on administration of recombinant factor VIII to keep factor VIII and PTT levels within acceptable limits.2,4
On the opposite spectrum of hematological conditions, sickle cell disease leads to increased coagulation with risks of thrombosis. Patients with sickle cell disease who undergo FTT are at an increased risk of microvascular thrombosis. In one case series, the rate of flap thrombosis was reported to be 33%, with partial and total flap loss found to be at 22% and 11%, respectively.5 While sickle cell trait has a relatively lower risk of complications, it is still an important consideration in flap surgery. There are few reports of successful free flap transfer in sickle cell trait.5 Improving flap survival in sickle cell trait has been reported by minimizing physiologic stress and optimizing hemodynamics by flap warming, use of antiplatelet and heparin solutions, high concentration oxygen, analgesia, and exchange transfusion.5
The combination of hemophilia A and sickle cell trait in a patient is rare.6 The risk of bleeding in hemophilia must be balanced against the risk of flap thrombosis with sickle cell trait. In this case, we report successful management of a patient with both hemophilia A and sickle cell trait who required FTT for an open ankle fracture complicated by a septic joint. Parallel management of severe hemophilia and sickle cell trait in the perioperative period is highlighted in the case discussion.

Methods
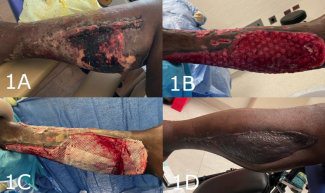
A 19-year-old male patient with severe hemophilia and sickle cell trait was injured in a motor vehicle collision and sustained severe bilateral lower extremity injuries. The patient underwent external fixation of his left knee for knee dislocation, which was complicated by compartment syndrome and required fasciotomies, serial debridements, and, ultimately, skin grafting. He also had an open right ankle fracture dislocation for which he underwent initial debridement with primary closure and plating of his lateral malleolus fracture. He required serial debridements of the right ankle wound, which evolved from a Gustilo 3A injury into a 3B injury with a large medial ankle soft tissue defect and infection with Enterobacter cloacae 1 week after open reduction and fixation (Figure 1).
Throughout the hospitalization, hematology and infectious disease were involved in patient care. The patient had been receiving 6000 units of Factor VIII (Nuwiq) twice weekly at home. On admission, the hematology team was consulted and emergently ordered 50 U/kg of factor VIII (Hemofil-M High; Takeda Pharmaceuticals) to be administered during the patient’s fasciotomy. Hematology further recommended the patient receive 25 U/kg factor VIII every 12 hours with daily monitoring of factor VIII levels and activated partial thromboplastin time (aPTT) to achieve a target factor VIII level >50% of normal, with instructions to follow up with their team before surgical intervention. With factor VIII levels and aPTT in the appropriate range, the patient’s coagulation profile was effectively normalized, a practice supported in the hemophilia flap literature.2 Electrophoresis was conducted to evaluate his sickle cell trait and found his hemoglobin (HbA) and sickle hemoglobin (HbS) at 75% and 22% respectively, which did not require further management.

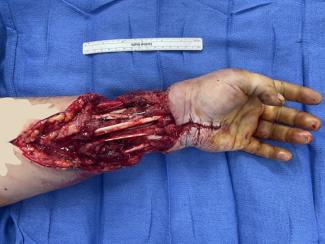
To provide definitive coverage of the right ankle, a gracilis free flap was done. The hematology team was available for recommendations in the case of intraoperative microvascular complications. Flap warming, use of antiplatelet and heparin solutions, high concentration oxygen, and analgesia were utilized.5 The flap was flushed with heparinized saline, and there was appropriate flow through the pedicle artery into the veins with minimal resistance. The health of the flap was confirmed clinically after end-to-end arterial and venous anastomosis, which proceeded without complication. Ischemia time was less than 1 hour (Figure 2).
Once the health of the flap was confirmed, the soft tissues over the joint were aggressively debrided. A healthy flap was inset over the anterior arthrotomy and tibia. Topical vancomycin and cefepime powder were placed in the joint, and resorbable antibiotic beads were placed under the flap over the soft tissues. Hemostasis was achieved with cautery. A split-thickness skin graft was harvested from the thigh and placed over the muscle. The proximal muscle was left uncovered as a monitor. A negative-pressure sponge was placed as a bolster and to minimize postoperative swelling over the flap; the sponge was left for 5 days at 75 mm Hg. The flap was monitored using a pencil Doppler over the vacuum tape to auscultate arterial and venous signals.
Late growth persisted from the joint cultures despite negative fibular hardware cultures, not an unusual scenario in limb salvage patients.7 Postoperatively, the patient’s factor VIII levels remained normalized with factor VIII repletion, and deep venous thrombosis (DVT) chemoprophylaxis with enoxaparin 30 mg every 12 hours was administered using standard post-traumatic dosing. Aspirin 81 mg was started on postoperative day 1 and continued for 1 month. Factor VIII and aPTT levels were monitored daily to ensure factor VIII remained >50% of normal, and the hematology team was in agreement with our chemoprophylaxis plan. DVT prophylaxis with enoxaparin was continued until the patient was no longer bedbound. Dangling was initiated 10 days postoperatively, and weight-bearing was restricted as per orthopedic recommendations.
Results
On follow-up 5 months after surgery, radiographic fracture healing in the ankle was observed, and the patient was weight-bearing on this extremity with no wound. Suppressive antibiotics were still being administered.
Discussion
Patients with hematological syndromes who require major surgery need frequent observation throughout their hospital stay. This patient was monitored successfully with routine hourly flap checks for several days, Doppler monitoring over the vacuum tape, and visual assessment to ensure proper healing.8 The negative-pressure dressing allowed for sterile coverage and edema control over the ankle.9 Close monitoring in an established flap monitoring unit is critical to ensuring proper healing postoperatively.10
To address the patient’s hematological conditions, the patient’s factor VIII levels were controlled in the preoperative period, making it unnecessary to make gross changes in factor VIII repletion postoperatively. With normal factor VIII and aPTT levels, the patient had effectively normal coagulation profiles and was deemed a candidate for the use of intraoperative heparinized saline and postoperative aspirin and enoxaparin. Enoxaparin primarily inactivates factor Xa, acting on a different step of the coagulation cascade than the repletion of factor VIII. Coordination with the patient’s hematological team ensured proper surveillance to effectively manage his bleeding profile during this high-risk time and to prevent overcorrection that would have led to thrombosis. If the patient’s factor VIII levels were less than 50% of normal, enoxaparin and aspirin would have been held, although this did not take place in this patient’s case. Hematology team consultation was a critical component in the careful balance to manage prothrombotic and procoagulant conditions.
Other precautions tailored to mitigate this patient’s risk of microvascular thrombosis included the use of warm fluids, a warming blanket over the flap, frequent flap monitoring, and conservative progression to flap dangling. Exchange transfusion could have been pursued if sickle cell hemoglobin concentrations were high. Technical considerations for free flap execution include the use of dual venous outflow, topical vasodilators, and pre- and intraoperative vascular testing.11-14
While a conservative strategy for wound coverage was considered, free tissue coverage permitted aggressive debridement of contaminated soft tissues and preservation of the implanted orthopedic hardware in this patient with an already extensively traumatized contralateral lower extremity.15 Coverage was pursued despite persistent positive cultures, a practice which is controversial. Though lower extremity coverage has been reported in wounds with positive cultures, it has been done with variable success.16,17 In cases where serial debridement is performed or irrigation dressing systems are implemented, high rates of salvage have been reported.16,17 Our group elected not to implant an irrigating vacuum system over the exposed ankle joint. Despite positive final post-debridement cultures at the time of coverage, the joint space had been thoroughly irrigated and debrided arthroscopically. Further discussion with regards to the management of contaminated or infected joint space is beyond the scope of this case report.
This patient with both severe hemophilia A and sickle cell trait was able to be successfully treated by a multidisciplinary team approach and with frequent monitoring of both the flap and his coagulation profile. Follow-up at 5 months was reassuring as the flap is healing well and the patient is weight-bearing on that extremity.
The practice of multidisciplinary patient care can be employed to address complex hematologic concerns commonly seen in preoperative microsurgery, such as in patients receiving direct anticoagulants, such as apixaban, or in patients with protein C and protein S deficiencies. Apixaban has emerged as a medication of choice for treatment and prevention of thromboembolic disorders in recent years, given that laboratory testing and medication dose titration are not typically necessary. As with warfarin in the preoperative setting, apixaban can be discontinued, and patients can receive perioperative bridging therapy with low-molecular-weight heparin or heparin infusions.18 In particular, patients with protein C and S deficiencies require delicate procoagulant management because use of traditional anticoagulation drugs like warfarin may, in fact, result in coagulation and skin necrosis, conditions which can complicate microsurgical procedures.19 In these patients, low-molecular-weight heparin or unfractionated heparin bridging may be beneficial in the intraoperative setting, and longer-term use of apixiban may be preferred postoperatively. Given the complexity of these hematologic conditions in small vessel reconstructive surgery patients, the plastic/microsurgeon is clearly well-served by seeking hematologic consultation before proceeding with surgery.20
Acknowledgments
Affiliations: 1The University of Queensland, Ochsner Clinical School, New Orleans, Louisiana; 2Intermountain Medical Center, Salt Lake City, Utah; 3Department of Orthopedic Surgery, Ochsner Health, New Orleans, Louisiana
Correspondence: Anna Garbuzov; garbuzov.anya@gmail.com
Ethics: This article complies with the Declaration of Helsinki.
Disclosures: The opinions expressed in this report are the authors’ own and are not the official position of the institutions represented. The authors have no relevant financial or nonfinancial interests to disclose.
References
1. Grant DW, Mlodinow A, Halen JPV, Kim JYS. Catastrophic outcomes in free tissue transfer: a six-year review of the NSQIP database. Plastic Surg Int. 2014;2014:1-10. doi:10.1155/2014/704206
2. Manickavachakan N, Ellur S, Joseph V, Victor J, Ross C. Flap cover in a patient with severe haemophilia type A. Indian J Plastic Surg. 2017;50(02):213-216. doi:10.4103/ijps.ijps_214_16
3. Lee BK, Shim JS. A case of heel reconstruction with a reverse sural artery flap in a hemophilia B patient. Archives Plastic Surg. 2012;39(2):150-153. doi:10.5999/aps.2012.39.2.150
4. Özkan Ö, Chen H-C, Mardini S, et al. Microvascular free tissue transfer in patients with hematological disorders. Plast Reconstr Surg. 2006;118(4):936-944. doi:10.1097/01.prs.0000232371.69606.61
5. Han KD, DeFazio MV, Lakhiani C, Evans KK. Free tissue transfer in patients with sickle cell trait. Plast Reconstr Surg. 2015;136(5):723e-725e. doi:10.1097/prs.0000000000001676
6. Maataoui HE, Fahi A, Oukkache B. Sickle cell trait and haemophilia: a rare association. Pan Afr Medical J. 2018;29:92. doi:10.11604/pamj.2018.29.92.14551
7. Zolper EG, Bekeny JC, Ormiston LD, et al. Day-of–free tissue transfer qualitative cultures do not predict limb salvage outcomes. Plastic Reconstr Surg. 2021;147(2):492-499. doi:10.1097/prs.0000000000007575
8. Lohman R, Langevin C-J, Bozkurt M, Kundu N, Djohan R. A prospective analysis of free flap monitoring techniques: physical examination, external Doppler, implantable Doppler, and tissue oximetry. J Reconstr Microsurg. 2013;29(01):051-056. doi:10.1055/s-0032-1326741
9. Chim H, Zoghbi Y, Nugent AG, Kassira W, Askari M, Salgado CJ. Immediate application of vacuum assisted closure dressing over free muscle flaps in the lower extremity does not compromise flap survival and results in decreased flap thickness. Arch Plast Surg. 2018;45(1):45-50. doi:10.5999/aps.2016.01977
10. Cornejo A, Ivatury S, Crane C, Myers J, Wang H. Analysis of free flap complications and utilization of intensive care unit monitoring. J Reconstr Microsurg. 2013;29(07):473-480. doi:10.1055/s-0033-1345434
11. Stranix JT, Anzai L, Mirrer J, et al. Dual venous outflow improves lower extremity trauma free flap reconstructions. J Surg Res. 2016;202(2):235-238. doi:10.1016/j.jss.2016.03.001
12. Stranix JT, Lee Z, Anzai L, et al. Optimizing venous outflow in reconstruction of Gustilo IIIB lower extremity traumas with soft tissue free flap coverage: Are two veins better than one? Microsurg. 2018;38(7):745-751. doi:10.1002/micr.30271
13. Haddock N, Garfein E, Saadeh P, Levine J. The lower-extremity Allen test. J Reconstr Microsurg. 2009;25(07):399-403. doi:10.1055/s-0029-1220861
14. Turin S, Walton R, Dumanian G, Hijjawi J, LoGiudice J, Alghoul M. Current practices in the management of postoperative arterial vasospasm in microsurgery. J Reconstr Microsurg. 2017;34(04):242-249. doi:10.1055/s-0037-1612601
15. Bonnevialle P. Operative treatment of early infection after internal fixation of limb fractures (exclusive of severe open fractures). Orthop Traumatology Surg Res. 2017;103(1):S67-S73. doi:10.1016/j.otsr.2016.06.019
16. Kurlander DE, Swanson M, Wee C, Knackstedt R, Gatherwright J. Flap plus sub-flap irrigation and negative pressure therapy for infected extremity wounds. Orthoplast Surg. 2020;1:16-20. doi:10.1016/j.orthop.2020.10.002
17. Kanuri A, O’Kelly ND, Shuck J, Kim P, Evans KK, Attinger CE. The effect of positive postdebridement cultures on local muscle flap reconstruction of the lower extremity. Plast Reconstr Surg Glob Open. 2018;6(9):e1864. doi:10.1097/gox.0000000000001864
18. Moster M, Bollinger D. Perioperative guidelines on antiplatelet and anticoagulant agents: 2022 update. Curr Anesthesiol Rep. 2022;12:286-296.
19. Ayala C, Blackwell K. Protein C deficiency in microvascular head and neck reconstruction. Laryngoscope.1999;109(2 Pt 1):259-265. doi:10.1097/00005537-199902000-00016
20. Pannucci CJ, Kovach SJ, Coker A. Microsurgery and the hypercoagulable state: a hematologist’s perspective. Plast Reconstr Surg. 2015 Oct;136(4):545e-552e. doi:10.1097/PRS.0000000000001591