Radiation-Induced Morphea of the Breast Treated With Wide Local Excision and Abdominal Free Flap Breast Reconstruction
© 2023 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of ePlasty or HMP Global, their employees, and affiliates.
Abstract
Radiation-induced morphea (RIM) associated with breast cancer treatment is a rare and underdiagnosed skin complication of radiotherapy that can lead to severe and painful contractures, resulting in disfigurement, failure of reconstruction, and poor quality of life in patients. The condition may present on a spectrum of local or more generalized forms involving skin over the breast and anterior chest wall. This diagnosis must be differentiated from post-radiation fibrosis, infection, cancer recurrence, inflammatory breast cancer, and other inflammatory conditions as the clinical course and treatment approaches differ. Various noninvasive and topical agents have been used; however, many cases are refractory to treatment. Surgery has been less commonly described in the management of generalized RIM. This report describes a case of RIM in a patient with breast cancer who experienced simultaneous resolution of symptoms as well as successful breast reconstruction using autologous free-tissue transfer.
Introduction
Radiotherapy is a major treatment modality for breast cancer, but it can result in skin changes in greater than 90% of cases.1-3 A rare underdiagnosed skin complication of radiation therapy is radiation-induced morphea (RIM).1,4,5,6 RIM is a local and chronically progressive radiation-associated scleroderma of the skin that leads to skin thickening and fibrosis, hyperpigmentation, and often painful disfigurement.Prompt diagnosis of this condition is crucial in order to halt or slow progression of RIM pathogenesis.3 There are limited descriptions of surgical management and breast reconstruction in the setting of RIM.
This case report describes a patient who underwent breast-conserving surgery and developed RIM that was refractory to medical management and resulted in severe disfigurement, excruciating pain, and poor quality of life. We present successful autologous free-flap breast reconstruction to relieve symptoms and highlight pertinent findings in the literature.
Case Presentation
A 74-year-old female patient with history of bilateral breast augmentation 40 years ago presented with early-stage, mammogram-detected, 1.2-cm, triple-positive invasive ductal carcinoma (IDC) of the left breast that was treated with breast-conserving therapy, sentinel node biopsy, implant removal, and bilateral breast reduction at an outside facility. Pathology revealed 0.2 cm of IDC, ductal carcinoma in situ at the margins, and a sentinel node with isolated tumor cells. Letrozole therapy was initiated, and the patient underwent whole-breast radiation.

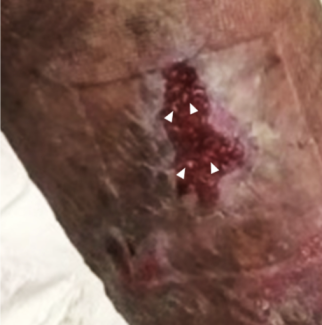
Seven months following radiation, she developed rapidly progressive skin changes, tightness, discoloration, loss of breast volume, and contracture within the left breast over 4 weeks. She was seen by breast surgery, dermatology, and oncology, and her care team discussed her presentation, imaging findings, and potential options for intervention. Results of imaging did not demonstrate concern for recurrent cancer; a diagnosis of RIM was tentatively made. Despite repeated triamcinolone injections, topical agents, and ultraviolet light therapy, her symptoms persisted. Her breast became severely deformed, woody, and excruciating painful, and the patient became unable to fully move her shoulder. Her nipple herniated through the constricting band of irradiated tissue. She presented 18 months following completion of radiation to our clinic (Figure 1). Her clinical examination and history were consistent with radiation-induced morphea, although no formal biopsy had been performed.

A key component for management of her symptoms and simultaneous breast reconstruction was a simple mastectomy and direct excision of as much radiated skin and subcutaneous tissue as possible, but this would result in a large chest wall defect. Because she had adequate lower abdominal tissue, a plan for a bipedicle deep inferior epigastric perforator flap was made for soft tissue coverage and to reconstruct the breast mound. The left breast’s irradiated skin and soft tissue was excised down to pectoralis muscle, which was unaffected, leaving a 15 × 15-cm defect. The recipient internal mammary vessels were prepared for microanastomosis; vessels did have some evidence of fibrosis, but this was not more extensive than what was expected. A bipedicle muscle-sparing abdominal-based free flap, based on medial row perforators, was harvested to maximize utility and perfusion of the entire lower abdominal tissue (Figure 2A). The entire flap was transposed to the chest wall, rotated 90 degrees, and then coned to provide both soft tissue coverage and breast mound projection, with the apex positioned to provide tissue for future nipple reconstruction (Figure 2B). The microanastomoses were performed to the anterograde and retrograde internal mammary vessels using both sets of vascular pedicles.

The patient’s 2-month postoperative results are shown in Figure 3. Her postoperative course was otherwise uncomplicated. Pathology revealed findings consistent with radiation-induced morphea including minimal fibrosis in the adipose tissue, extensive dermal sclerosis, thickened collagen fibers, and prominent inflammatory infiltrates in the subcutaneous layer (Figure 4). The patient reported relief of her symptoms, including pain relief, recovery in range of motion of her arm and shoulder, and ability to enjoy activities.

Discussion
RIM is a rare complication of radiotherapy, with symptoms typically presenting 3 to 12 months after exposure, but latent symptoms have been reported to present up to 30 years from exposure 4,7-9 Clinical presentation is nonspecific and can be confused with other conditions, such as chronic radiation dermatitis, fibrosis, and autoimmune disorders.7 Presentation has been described as discoloration of the areola/skin, erythematous plaques, linear lesions, skin thickening, peau d’orange, edema, and skin or fat sclerosis.5,8-11
Morphea is a dermatologic and rheumatologic condition characterized by thickened collagen and abnormal activity of fibroblasts.4 The underlying pathogenesis is poorly understood and may involve an inflammatory process involving transforming growth factor-beta secretion.7,10 A key difference between RIM and postradiation fibrosis is that RIM involves only dermal fibrosis, while radiation fibrosis mainly affects the subcutaneous and fascial levels.4 Treatments for RIM have had limited success, including topical steroid ointments or injections and ultraviolet therapy. This patient underwent each of these treatments with no improvement. Treatments used in conventional morphea have also been used with little effect, including topical steroids, tacrolimus, and methotrexate.4,8,10 Surgery with reconstruction has rarely been published but has been shown to have satisfactory outcomes for relief of symptoms in refractory cases or generalized forms of RIM.12,13
Autologous reconstruction can be used following simple mastectomy to restore the breast mound. The use of perforator flaps has notable benefits in reduced donor site morbidity as described in the reconstructive literature, and the lower abdomen is considered a gold standard approach in breast reconstruction.14 The use of a bipedicle flap allowed use of all 4 zones of perfusion in the lower abdomen.15 Specifically, given the extensive nature of the grossly abnormal tissue, the decision was made preoperatively to perform a bipedicled flap in order to utilize the entire abdominal tissue to reconstruct the anticipated skin defect after resection. A bipedicled flap (as opposed to a traditional unipedicle flap) allows for more abdominal tissue to be reliably perfused due to the nature of the dual blood supply. Because of the poor response to medical management, radical surgical excision seemed to be the logical next step in managing this patient’s symptoms. The use of the abdominal free flap with dual bipedicle blood supply provided ample soft tissue coverage and allowed for reconstruction of a natural-appearing breast mound. An important technical note is that the insetting of the flap in cone-type fashion mimics the natural projection of a patient’s native breast. Postoperatively, this patient was followed very closely, being seen every few weeks initially after surgery with a plan to follow her every 6 months thereafter.
Conclusions
Our case highlights the importance of early recognition with close follow-up for patients with RIM. Without early recognition, there can be continued disease progression and possibly worsened severity of symptoms for a longer period of time. In severe forms, more radical approaches may be required, involving complete excision of involved tissue. The armamentarium of the plastic surgeon offers various reconstruction options that can lead to the alleviation of symptoms associated with RIM and improved quality of life.
Acknowledgments
Ashley Titan, MD, and Anita T. Mohan, MBBS, PhD, MBA, were joint first authors of this manuscript.
Affiliations: 1Division of Plastic Surgery, Stanford University School of Medicine, Stanford, California; 2Department of Pathology, Stanford University School of Medicine, Stanford, California
Correspondence: Gordon K Lee, MD; glee@stanford.edu
Ethics: Informed consent was obtained from the patient included in the study. Also, written informed consent for patient information and images to be published was provided by the patient.
Disclosures: The authors have no relevant financial or nonfinancial interests to disclose.
References
1. Harper JL, Franklin LE, Jenrette JM, Aguero EG. Skin toxicity during breast irradiation: pathophysiology and management. South Med J. 2004;97(10):989-993. doi:10.1097/01.SMJ.0000140866.97278.87
2. Bleasel NR, Stapleton KM, Commens C, Ahern VA. Radiation-induced localized scleroderma in breast cancer patients. Australas J Dermatol. 1999;40(2):99-102. doi:10.1046/J.1440-0960.1999.00330.X
3. Senkus-Konefka E, Jassem J. Complications of Breast-cancer radiotherapy. Clin Oncol. 2006;18(3):229-235. doi:10.1016/J.CLON.2005.11.004
4. Spalek M, Jonska-Gmyrek J, Gałecki J. Radiation-induced morphea - a literature review. J Eur Acad Dermatol Venereol. 2015;29(2):197-202. doi:10.1111/JDV.12704
5. Partl R, Regitnig P, Tauber G, Pötscher M, Bjelic-Radisic V, Kapp KS. Radiation-induced morphea—a rare but severe late effect of adjuvant breast irradiation: Case report and review of the literature. Strahlentherapie Und Onkologie. 2018;194(11):1060. doi:10.1007/S00066-018-1336-9
6. Dyer BA, Hodges MG, Mayadev JS. Radiation-induced morphea: An under-recognized complication of breast irradiation. Clin Breast Cancer. 2016;16(4):e141-e143. doi:10.1016/j.clbc.2016.05.001
7. Shetty G, Lewis F, Thrush S. Morphea of the breast: Case reports and review of literature. Breast J. 2007;13(3):302-304. doi:10.1111/J.1524-4741.2007.00427.X
8. Schaffer J v., Carroll C, Dvoretsky I, Huether MJ, Girardi M. Postirradiation morphea of the breast presentation of two cases and review of the literature. Dermatology. 2000;200(1):67-71. doi:10.1159/000018322
9. Dubner S, Bovi J, White J, Susnik B. Postirradiation morphea in a breast cancer patient. Breast J. 2006;12(2):173-176. doi:10.1111/J.1075-122X.2006.00229.X
10. Friedman O, Barnea Y, Hafner A. Underdiagnosed and disfiguring - Radiation-induced morphea following breast cancer treatment. The Breast. 2018;39:97-100. doi:10.1016/J.BREAST.2018.04.006
11. Schaffer J v., Carroll C, Dvoretsky I, Huether MJ, Girardi M. Postirradiation morphea of the breast presentation of two cases and review of the literature. Dermatology. 2000;200(1):67-71. doi:10.1159/000018322
12. Dancey AL, Waters RA. Morphea of the breast. Two case reports and discussion of the literature. J Plast Reconstr Aesthet Surg. 2006;59(10):1114-1117. doi:10.1016/J.BJPS.2006.01.018
13. Rafique B, McInerney N, Fitzgerald G, O’Hanlon D, Gilmore J, Kelly EJ. Post-irradiation morphea of the breast: does this pose an issue for reconstruction? Eur J Plast Surg. 2017;40(1):67-70. doi:10.1007/S00238-016-1226-2/FIGURES/3
14. Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg. 1993;32(1):32-38. http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=Retrieve&list_uids=8141534&dopt=abstractplus\npapers://76e45e2a-1c06-45ff-bec5-2d0d34b35934/Paper/p4730
15. Boyd JB, Taylor GI, Corlett R. The vascular territories of the superior epigastric and the deep inferior epigastric systems. Plast Reconstr Surg. 1984;73(1):1-16. doi:10.1097/00006534-198401000-00001