Split-Thickness Skin Graft Donor Sites
Questions
- What is the role of epidermal stem cells in the healing of a split-thickness skin graft donor site?
- What are the principles regarding the harvesting of a split-thickness skin graft?
- What morbidities can occur following the harvesting of a split-thickness skin graft?
- How are these donor sites best dressed?
Case Description
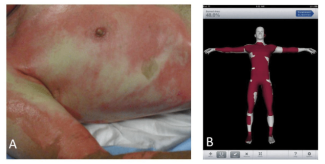
A 22-year-old male sustained third-degree burns to his right arm in a house fire. The burn was excised and resurfaced with a split-thickness skin graft. The donor site was dressed with Xeroform and bacitracin. The dressing adhered to the wound and over 2 weeks separated spontaneously as the wound epithelialized (Figure 1).

Q1. What is the role of epidermal stem cells in the healing of a split-thickness skin graft donor site?
Since the size of the donor site limits wound closure from the wound edges, re-epithelialization relies on the regenerative capacity of populations of epidermal stem cells (ESCs) in the residual dermal appendages. Many different stem cell populations have been shown to induce skin repair since the interfollicular epidermis (IFE) requires constant renewal, and hair follicle stem cells undergo a cycle of phases.Under normal conditions, several stem cell populations reside in specific microenvironments, called stem cell niches, that replenish a discrete compartment of the skin (Figure 2).1

The IFE is maintained by unipotent stem cells in the basal layer of the epidermis. They give rise to short-lived progenitors that produce epidermal proliferative units that differentiate to maintain a mature epidermis.2 A major stem cell reservoir in the outer root sheath of the hair follicle below the sebaceous gland is called the follicular bulge and contributes to the maintenance of the follicle. Here the 7-transmembrane leucine-rich G-protein coupled receptor 5 (Lgr5) is a marker of the Wnt-regulated adult stem cell population. Also identified is Lgr6 as a marker of progenitors in a Wnt-independent niche, just above the follicular bulge, in the isthmus region. One-third of these cells also express marker proteins MGS24 and Lrig1, which contribute to the infundibulum that interfaces with the IFE, and a few co-express the transcription factor Blimp1, associated with the maintenance of sebaceous gland cells.3 Thus, Lgr6 stem cells contribute to the infundibulum, the isthmus, and the cells of the sebaceous gland.4
The donor site is devoid of IFE. When the skin is injured, however, these two follicular stem cell populations are actively mobilized to produce all the cell lineages of the skin including the sebaceous gland and the IFE, the process requiring complex epithelial-mesenchymal interactions.5,6

Q2. What are the principles regarding the harvesting of a split-thickness skin graft?
Consideration is first given to the compatibility of size, color, texture, and hair density of the donor site with that of the defect. The operation is usually performed under general anesthesia; however, long-acting local anesthetic infiltration (0.25% Marcaine with epinephrine) or regional nerve blocks may be used in selected patients. Power-driven dermatomes are most commonly used to harvest split-thickness skin grafts (Figure 1), although calibrated hand-driven instruments such as a Humby knife can be used (Figure 3). The thighs and buttocks provide convex surfaces that facilitate the procedure, and scar and pigmentary changes can be concealed by clothing. Grafts taken from above the clavicle provide a superior color match for facial defects, and the scalp donor scar is imperceptible following hair growth.The back is favored in the elderly because the skin is thick enough to reliably heal.7 The anterior chest wall, upper extremities, and legs offer scraps of skin that may be useful only for very small defects. Infiltration of saline over the ribs, spine, or iliac crest facilitates harvesting over bony prominences. In large wounds, when donor skin is scarce, unusual donor sites such as the scrotum or axilla can provide useful skin following tumescence. Since the dermis never regenerates, the number of times a site can be harvested will depend on the thickness of the donor site and the depth at which the graft is taken.
As for the technique, the dermatome abruptly contacts the skin at an angle of 45 degrees and is guided forward, exerting a steady downward pressure against the assistant’s countertraction. The cut graft can be allowed to accumulate in the pocket of the handpiece, although some surgeons prefer to use tissue forceps to gently lift the graft as it emerges. When an adequate amount of skin has been cut, the leading edge is severed from the donor site by an upward movement with the dermatome still running. The dermatome is then released, and the skin graft retrieved.8
Q3. What morbidities can occur following the harvesting of a split-thickness skin graft?
Harvesting a split-thickness skin graft creates a partial-thickness wound that is burdened with a number of morbidities. Early problems include distressing pain and itching from the exposure of nerve endings. Although these symptoms seldom last more than 21 days,9 they can prevent mobilization and sleep and demand frequent analgesic and antipruritic agents (Figure 4A and C). Continued scratching can also tear the newly healed epithelium and promote infection. Sinha et al. recommended several evidence-based strategies to palliate donor site pain, which included: (a) subcutaneous local anesthetic injection (Lidocaine 1:500,00 epinephrine); (b) topical agents (2% lidocaine and 0.5% bupivacaine); (c) ice; and (d) hydrocolloid and polyurethane-based wound dressings.10

Infection tends to be local and responds well to wound care and topical and systemic antibiotics but can delay wound healing, cause scarring, and convert the wound to a full-thickness injury. Leakage of hematoma and exudate beneath bio-occlusive dressings (Figure 4B) can cause patient distress. Skin discoloration is a common causes of patient dissatisfaction.9 An initial hypopigmentation often progresses to dyschromia (hyperpigmentation interspersed with patches of hypopigmentation), and at 6 months post surgery the skin becomes hyperpigmented (Figure 4C and D).11 Donor sites are expected to heal within 2 to 3 weeks, though this can exceed a month for thicker grafts (>0.12 inch) due to fewer available skin appendages. This tends to produce hypertrophic scars, especially in children and those with dark skin, causing poor psychological and aesthetic outcomes, a factor that must be considered when choosing surgical sites (Figure 4D). Care must be taken to avoid harvesting at the wrong depth by injudicious use of the dermatome, which can expose vital structures and non-vascularized tissue, necessitating corrective reconstructive procedures. A rare complication is the development of squamous cell carcinoma. 12
Q4. How are these donor sites best dressed?
The ideal donor dressing should encourage wound healing, minimize pain, impair infection, and reduce scarring. Large donor sites are conveniently covered with nonmoist dressings and include fine mesh gauze impregnated with Vaseline (Jelonet), an antibacterial agent such as 3% bismuth tribromophenate (Xeroform), and o-tolylazo-o-tolylazo-beta-naphthol (Scarlet Red). These dressings adhere to the wound and separate spontaneously as the wound re-epithelializes. Their main drawback is pain during the first few days. The discomfort of the first postoperative dressing change can be minimized by placing a layer of non-adherent gauze (eg, Telfa) between the dressing and the outer compression bandage. Biobrane, consisting of collagen peptides integrated into a knitted nylon fabric, is more comfortable than Xeroform but is associated with a higher infection rate. Nonmoist dressings require minimum nursing care, with healing occurring predictably in 7 to 10 days and, apart from Biobrane,are relatively inexpensive.
For smaller wounds, non-adherent moist dressings provide optimum conditions for epithelial proliferation, migration, and angiogenesis. They are categorized as occlusive dressings, such as a hydrocolloid fiber dressing (eg, Duoderm) and semiocclusive polyurethane films (eg, Opsite and Tegaderm). Semiocclusive dressings are impermeable to bacteria and liquids, and the weeping of exudate from beneath the dressing in the first 24 hours complicates management. A recent meta-analysis indicated pain scores and re-epithelialization rates were improved with moist compared with nonmoist dressings, but there was no evidence to support the superiority of one category concerning infection rates or cosmetic outcomes.13 The benefit of antimicrobial silver-impregnated dressings, such as nanocrystalline silver (Acticoat) or hydrofiber (Aquacel Ag), in reducing bacterial growth remains controversial. Two prospective comparative studies comparing Acticoat with Allevyn, a polyurethane dressing, demonstrated no statistical difference in microbial growth and Acticoat was less painful.14
Acknowledgments
Affiliations: Professor of Plastic and Reconstructive Surgery, Johns Hopkins University School of Medicine, Retired, Baltimore MD; Director, Johns Hopkins Burn Center, Retired, Baltimore, MD
Correspondence: Stephen M Milner, MBBS, BDS, DSc (Hon) FRCSE, FACS; stephenmilner123@gmail.com
Disclosure: The authors disclose no financial or other conflicts of interest.
References
1. Blanpain C, Fuchs E. Epidermal Stem Cells of the Skin. Annu Rev Cell Dev Biol. 2006;22(1):339-373. doi:10.1146/annurev.cellbio.22.010305.104357
2. Clayton E, Doupé DP, Klein AM, Winton DJ, Simons BD, Jones PH. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446(7132):185-189. doi:10.1038/nature05574
3. Horsley V, O’Carroll D, Tooze R, et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597-609. doi:10.1016/j.cell.2006.06.048
4. Haegebarth A, Clevers H. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol. 2009;174(3):715-721. doi:10.2353/ajpath.2009.080758
5. Snippert HJ, Haegebarth A, Kasper M, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327(5971):1385-1389. doi:10.1126/science.1184733
6. Leushacke M, Barker N. Lgr5 and Lgr6 as markers to study adult stem cell roles in self-renewal and cancer. Oncogene. 2012;31(25):3009-3022. doi:10.1038/onc.2011.479
7. Heimbach DM, Engrav LH. Surgical Management of the Burn Wound. Raven Press; 1984.
8. Milner SM. Skin grafting. In: Evans G, ed. Operative Plastic Surgery. 2nd ed. Oxford University Press; 2019:65-72. Accessed December 12, 2022. doi.org/10.1093/med/9780190499075.003.0007
9. Asuku M, Yu TC, Yan Q, et al. Split-thickness skin graft donor-site morbidity: A systematic literature review. Burns. 2021;47(7):1525-1546. doi:10.1016/j.burns.2021.02.014
10. Sinha S, Schreiner AJ, Biernaskie J, Nickerson D, Gabriel VA. Treating pain on skin graft donor sites: Review and clinical recommendations. J Trauma Acute Care Surg. 2017;83(5):954-964. doi:10.1097/TA.0000000000001615
11. Otene C, Olaitan PB, Ogbonnaya IS, Nnabuko RE. Donor site morbidity following harvest of split-thickness skin grafts in South Eastern Nigeria. J West Afr Coll Surg. 2011;1(2):86-96.
12. Thomas W, Rezzadeh K, Rossi K, Shah A. Squamous Cell Carcinoma Arising at a Skin Graft Donor Site: Case Report and Review of the Literature. Plast Surg Case Stud. doi:10.1177/2513826X211008425
13. Serebrakian AT, Pickrell BB, Varon DE, et al. Meta-analysis and Systematic Review of Skin Graft Donor-site Dressings with Future Guidelines. Plast Reconstr Surg Glob Open. 2018;6(9):e1928:1-9. doi:10.1097/GOX.0000000000001928
14. Argirova M, Hadjiski O, Victorova A. Acticoat versus Allevyn as a split-thickness skin graft donor-site dressing: A prospective comparative study. Ann Plast Surg. 2007;59(4):415-422. doi:10.1097/SAP.0b013e3180312705
References
1. Blanpain C, Fuchs E. Epidermal Stem Cells of the Skin. Annu Rev Cell Dev Biol. 2006;22(1):339-373. doi:10.1146/annurev.cellbio.22.010305.104357
2. Clayton E, Doupé DP, Klein AM, Winton DJ, Simons BD, Jones PH. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446(7132):185-189. doi:10.1038/nature05574
3. Horsley V, O’Carroll D, Tooze R, et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597-609. doi:10.1016/j.cell.2006.06.048
4. Haegebarth A, Clevers H. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol. 2009;174(3):715-721. doi:10.2353/ajpath.2009.080758
5. Snippert HJ, Haegebarth A, Kasper M, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327(5971):1385-1389. doi:10.1126/science.1184733
6. Leushacke M, Barker N. Lgr5 and Lgr6 as markers to study adult stem cell roles in self-renewal and cancer. Oncogene. 2012;31(25):3009-3022. doi:10.1038/onc.2011.479
7. Heimbach DM, Engrav LH. Surgical Management of the Burn Wound. Raven Press; 1984.
8. Milner SM. Skin grafting. In: Evans G, ed. Operative Plastic Surgery. 2nd ed. Oxford University Press; 2019:65-72. Accessed December 12, 2022. doi.org/10.1093/med/9780190499075.003.0007
9. Asuku M, Yu TC, Yan Q, et al. Split-thickness skin graft donor-site morbidity: A systematic literature review. Burns. 2021;47(7):1525-1546. doi:10.1016/j.burns.2021.02.014
10. Sinha S, Schreiner AJ, Biernaskie J, Nickerson D, Gabriel VA. Treating pain on skin graft donor sites: Review and clinical recommendations. J Trauma Acute Care Surg. 2017;83(5):954-964. doi:10.1097/TA.0000000000001615
11. Otene C, Olaitan PB, Ogbonnaya IS, Nnabuko RE. Donor site morbidity following harvest of split-thickness skin grafts in South Eastern Nigeria. J West Afr Coll Surg. 2011;1(2):86-96.
12. Thomas W, Rezzadeh K, Rossi K, Shah A. Squamous Cell Carcinoma Arising at a Skin Graft Donor Site: Case Report and Review of the Literature. Plast Surg Case Stud. doi:10.1177/2513826X211008425
13. Serebrakian AT, Pickrell BB, Varon DE, et al. Meta-analysis and Systematic Review of Skin Graft Donor-site Dressings with Future Guidelines. Plast Reconstr Surg Glob Open. 2018;6(9):e1928:1-9. doi:10.1097/GOX.0000000000001928
14. Argirova M, Hadjiski O, Victorova A. Acticoat versus Allevyn as a split-thickness skin graft donor-site dressing: A prospective comparative study. Ann Plast Surg. 2007;59(4):415-422. doi:10.1097/SAP.0b013e3180312705