Sunburn
© 2024 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of ePlasty or HMP Global, their employees, and affiliates.
Questions
1. What is the pathophysiology of sunburn and how does ultraviolet radiation modulate the inflammatory response?
2. How does the skin function as a barrier against ultraviolet radiation?
3. How should sunburn be treated?
4. What is the evolutionary benefit of skin pigmentation?
Case Description
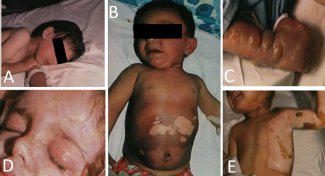
A 16-year-old healthy female sought care at the burn center due to sunburn. She presented with painful, red skin, predominantly over her back and shoulders, 24 hours after sunbathing without sun protection. The patient further experienced fever, nausea, and headache. Symptoms peaked at 12 hours. Following supportive care, including analgesics and rehydration, the condition resolved within 72 hours, leaving no residual scarring (Figure 1).

Figure 1. Sunburn. The erythema contrasts with the unaffected skin over the medial border of the right upper arm.
Q1. What is the pathophysiology of sunburn and how does ultraviolet radiation modulate the inflammatory response?
Sunburn results from excessive exposure to solar ultraviolet radiation (UVR). The principal cause is UVB, which elicits an inflammatory response within the epidermis. Despite the lack of significant radiant heat, it is classified as a superficial or first-degree burn. Patients present with the cardinal signs and symptoms of inflammation, with the skin being red, warm, swollen, and tender. Typically, this occurs 3 to 5 hours after sun exposure, peaks at 12 to 24 hours and, as the epidermis peels, resolves without scarring within a week. Severe cases may cause blistering and be complicated by dehydration and secondary infection.
UVR is a component of the electromagnetic spectrum. It constitutes 5% of solar radiation and comprises UVA (320-400 nm), UVB (280-320 nm), and UVC (100-280 nm).2 The depth of skin penetration correlates directly with the wavelength (Figure 2). UVB radiation has been shown to induce keratinocytes to release RNA, which interacts with Toll-like receptor 3, triggering the activation of nuclear factor kappa beta (NFκB) prompting the production of proinflammatory cytokines, including IL-6 and TNF-α.3 At high doses, UVB radiation triggers apoptosis in keratinocytes, referred to as sunburn cells, which are characterized by pyknotic nuclei.

Figure 2. The electromagnetic spectrum of UV radiation. The atmospheric ozone blocks UVC so that ambient sunlight is approximately 90% to 95% UVA, which penetrates the dermis. Five to ten percent is UVB, which is almost totally absorbed by the epidermis. Melanin acts as a photoprotective filter, attenuating the penetration of all wavelengths of light into the subdermal tissues.
UVB photons are absorbed by DNA, generating pyrimidine photoproducts, such as cyclobutane pyrimidine dimers. Failure of DNA repair mechanisms can induce mutations in proto-oncogenes and tumor-suppressor genes, increasing the risk of skin cancer.1 UVA can also promote carcinogenesis by the generation of reactive oxygen species (ROS), causing DNA and macromolecular damage. After a few hours, the damage response signals subside and the wound heals as keratinocytes proliferate under the influence of epidermal growth factors.2
Q2. How does the skin function as a barrier against ultraviolet radiation?
Ultraviolet radiation triggers a protective response in the skin through the production of melanin. There are two types of melanin: brown-black eumelanin and red-yellow pheomelanin. Melanin synthesis occurs within melanosomes, membrane-bound organelles, located in melanocytes in the basal layer of the epidermis. A single melanocyte interacts with 30 to 40 keratinocytes, forming an epidermal melanocyte unit, and transfers melanosomes through their dendrites.4 Keratinocytes contribute to this process by producing cytokines and growth factors that can modulate the proliferation and differentiation of melanocytes.5 Degranulation of melanosomes releases eumelanin that shields keratinocyte nuclei, absorbing UVR and dissipating the energy as heat by a quantum mechanical process called internal conversion.6 Pheomelanin provides less protection and is phototoxic, producing ROS when exposed to UVA.7
UVR-induced damage to keratinocyte DNA triggers the activation of transcription factor p53, which in turn enhances the expression of the protein prop-opiomelanocortin (POMC), a precursor to alpha-melanocyte–stimulating hormone (α-MSH). The binding of α-MSH to the melanocortin 1 receptor (MC1R) on the cell membrane of the melanocyte initiates a series of intracellular events. Subsequent changes in gene expression promote the production of enzymes and other proteins needed for melanin synthesis within the melanosomes (Figure 3).2

Figure 3. Signaling pathways in melanogenesis. α-MSH is synthesized by the anterior pituitary gland and binds to the G-protein coupled receptor, melanocortin 1 receptor (MC1R), which activates adenylate cyclase. Adenylate cyclase catalyzes the conversion of ATP to cAMP, which activates protein kinase A (PKA). PKA phosphorylates cellular targets, such as the transcription factor cyclic AMP response element binding protein (CREB). CREB, in turn, transcriptionally activates various genes including that encoding the protein microphthalmia-associated transcription factor (MITF) that is crucial to the synthesis of melanogenic enzymes. These are transported into the melanosomes for synthesis of melanin and delivery to the keratinocytes.
The rate-limiting step in melanin synthesis is the hydroxylation of tyrosine by tyrosinase (TYR) to L-3,4-dihydroxyphenylalanine (L-DOPA) and dopaquinone. L-DOPA is subsequently oxidized back to dopaquinone, after which the pathways diverge and are compartmentalized in eumelanosomes or pheomelanosomes. Dopachrome undergoes further enzymatic modifications involving tyrosine-related protein (TRP-1) and dopachrome tautomerase (TRP-2) to form melanin monomers that polymerize to form eumelanin. However, in the presence of cysteine, dopachrome yields cysteinyldopa intermediates, which undergo oxidative polymerization to pheomelanin (Figure 4).Human skin color is then determined by the ratio of eumelanin to total melanin.8 Pheomelanin predominance leads to the red hair fair skin phenotype.7

Figure 4. Simplified scheme of the biosynthesis of melanin. TYR, tyrosine; TRP-1, tyrosine-related protein;TRP-2, dopachrome tautomerase.
Q3. How should sunburn be treated?
The treatment of sunburn is primarily aimed at symptomatic relief. Such measures include the use of cool baths, compresses, and showers as well as opting for loose clothing. Emollients, such as aloe vera, can be employed for their soothing qualities. Ensuring the patient is well-hydrated is crucial, and in cases of secondary infection, antibiotics may be warranted.
A comprehensive review of 40 studies by Han et al evaluated commonly prescribed medications for sunburn.9 Notably, topical and oral nonsteroidal anti-inflammatory analgesics (NSAIDs), such as aspirin, ibuprofen, and indomethacin, have been suggested for their capacity to inhibit prostaglandins and alleviate pain. UV light exposure prompts an increase in prostaglandin E2 levels in the skin, contributing to erythema, and NSAIDs have been shown to reduce erythema for 24 to 36 hours. Topical and systemic corticosteroids regardless of the time of application, strength or dosage; antihistamines (H1 and H2 antagonists); antioxidants; aspirin; aloe vera; and vitamins had little or no effect on reducing recovery time. Ultimately, the most effective approach to sunburn continues to be prevention through physical means and the use of sunscreens.9
Q4. What is the evolutionary benefit of skin pigmentation?
The evolution of skin pigmentation represents a balance between the need to shield the skin from intense solar UVR and the ability to produce vitamin D. Around 2.8 million years ago (mya), hominins in Africa possessed lightly pigmented skin that could tan and was covered with dark hair. Approximately 2 mya, a shift in pigmentation coincided with the loss of body hair and an increase in eccrine sweat glands.10 This adaptation persisted through the emergence of Homo sapiens about 0.3 mya and was linked to the development of striding bipedal locomotion, increasing the need for thermoregulation through evaporative cooling and heat dissipation from the skin's surface.11
The subsequent establishment of dark skin was primarily driven by a selective sweep eliminating variation in the key melanocortin receptor gene (MC1R), and selection for multiple derived alleles, including dark pigment variants of DDB1 and MFSD12. Populations in high UVR regions show a scarcity of nonsynonymous mutations in the MC1R gene. Dark skin in equatorial regions is thought to be a protective adaptation against the harmful UVR, particularly UVB, through the production of eumelanin. Since sunburn and skin cancer typically occur after a reproductive age, melanized skin likely evolved to prevent UV-induced photolysis of folate, crucial for preventing neural tube defects and infertility.12
Around 80,000 years ago, depigmentation emerged as populations migrated northward, driven by the need for UVB-induced vitamin D biosynthesis, vital for bone metabolism, immune function, cellular growth, and childbirth.11,13 Fair-skinned individuals from Northern European and Asian populations exhibit greater MC1R gene diversity with abundant nonsynonymous variants. Genetic depigmentation variants such as SLC24A5, SLC45A2, MC1R, TYR, TYRP1, and OCA2 are associated with these populations. The SCL24A5 gene, encoding a cation exchanger in melanosomes, has swept to near fixation in Europeans and light-skinned KhoeSan populations of South Africa, where UVR is reduced.14,15
Acknowledgments
Author: Stephen M. Milner, MBBS, BDS, DSc (Hon), FRCSE, FACS
Affiliation: Professor of Plastic and Reconstructive Surgery, Johns Hopkins University School of Medicine, Baltimore, Maryland (Ret)
Correspondence: Stephen M. Milner, MBBS, BDS, DSc (Hon), FRCSE, FACS; stephenmilner123@gmail.com
Disclosures: The author has no financial or other conflicts of interest to disclose.
References
1. Sarasin A. The molecular pathways of ultraviolet-induced carcinogenesis. Mutat Res. 1999;428(1-2):5-10. doi:10.1016/s1383-5742(99)00025-3
2. D'Orazio JD, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14:12222-12248. doi:10.3390/ijms140612222
3. Bernard JJ, Cowing -Zitron C, Nakatsuji T, et al. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat Med. 2012;18(8):1-7. doi:10.1038/nm.2861.Ultraviolet
4. Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445(7130):843-850. doi:10.1038/nature05660
5. Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007;21(4):976-994. doi:10.1096/fj.06-6649rev
6. Ilena A, Thorn KE, Hume PA, Hodgkiss JM. The photoprotection mechanism in the black–brown pigment eumelanin. Proc Natl Acad Sci U S A. 2022;119(43):e2212343119. doi.org/10.1073/pnas.221234311
7. Yardman-Frank JM, Fisher DE. Skin pigmentation and its control: From ultraviolet radiation to stem cells. Exp Dermatol. 2021;30(4):560-571. doi:10.1111/exd.14260
8. Cichorek M, Wachulska M, Stasiewicz A, Tymińska A. Skin melanocytes: Biology and development. Postepy Dermatol Alergol. 2013;30(1):30-41. doi:10.5114/pdia.2013.33376
9. Han A, Maibach HI. Management of acute sunburn. Am J Clin Dermatol. 2004;5(1):39-47. doi:10.2165/00128071-200405010-00006
10. Jablonski NG. The evolution of human skin pigmentation involved the interactions of genetic, environmental, and cultural variables. Pigment Cell Melanoma Res. 2021;34(4):707-729. doi:10.1111/pcmr.12976
11. Jablonski NG, Chaplin G. Human skin pigmentation as an adaptation to UV radiation. Proc Natl Acad Sci U S A. 2010;107(SUPPL. 2):8962-8968. doi:10.1073/pnas.0914628107
12. Borradale D, Isenring E, Hacker E, Kimlin M. Exposure to solar ultraviolet radiation is associated with a decreased folate status in women of childbearing age. J Photochem Photobiol B. 2014;131:90-95. doi: 10.1016/j.jphotobiol.2014.01.002
13. Yuen AWC, Jablonski NG. Vitamin D: In the evolution of human skin colour. Med Hypotheses. 2010;74(1):39-44. doi:10.1016/j.mehy.2009.08.007
14. Feng Y, McQuillan MA, Tishkoff SA. Evolutionary genetics of skin pigmentation in African populations. Hum Mol Genet. 2021;30(R1):R88-R97. doi:10.1093/hmg/ddab007
15. Lin M, Siford RL, Martin AR, et al. Rapid evolution of a skin-lightening allele in southern African KhoeSan. Proc Natl Acad Sci U S A. 2018;115(52):13324-13329. doi:10.1073/pnas.1801948115