Aftermath of Sulfur Mustard Poisoning
Stephen M. Milner, MBBS, BDS, DSc (Hon), FRCSE, FACS
© 2024 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of ePlasty or HMP Global, their employees, and affiliates.
Questions
1. What are the biochemical mechanisms of sulfur mustard toxicity?
2. What are the clinical features of sulfur mustard injury?
3. How are casualties of sulfur mustard poisoning treated?
4. What are the long-term effects of sulfur mustard exposure?
On June 28, 1987, the densely populated city of Sardasht, in Northwestern Iran, sustained an Iraqi aerial bombardment of four 250-kg bombs containing sulfur mustard warheads (Figure 1).1

Figure 1. Chemical bombardment of Sardasht in Northwestern Iran in June 1987. (Photograph taken from Ghanei et al 2003, with permission from the Journal of Burns and Surgical Wound Care.)
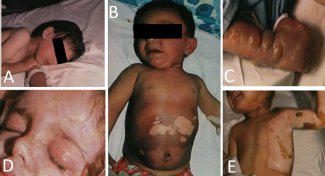
There were 4500 casualties, of which 3000 received outpatient treatment for mild sulfur mustard exposure. The remaining 1500 patients, exposed to higher concentrations, experienced moderate to severe complications requiring hospitalization The most significant medical repercussions were observed in infants and children, who endured persistent chronic health issues 14 years after the attack (Figure 2).1

Figure 2. Casualties of Sardasht showing early features of sulfur mustard exposure. (A) Erythema of the face in a 4-year-old boy. (B) A 10-month-old boy with darkening of the skin of the abdomen. The blotchy areas of hypopigmentation follow desquamation. (C) Multiple blisters on the leg. (D) Swelling of the upper eyelids. (E) Hypopigmentation of the chest left axilla and arm. (Photograph taken from Ghanei et al 2003, with permission from Journal of Burns and Surgical Wound Care.)
Q1. What are the biochemical mechanisms of sulfur mustard toxicity?
Sulfur Mustard (SM), bis (2-chlorodiethyl) sulfide, is a powerful alkylating agent with electrophilic properties. It forms covalent adducts with macromolecules, primarily DNA, proteins, and cell membrane components. The formation of DNA cross-links and strand breaks can trigger repair processes, cause cell death, inhibit cell division, alter gene expression, and disrupt multiple cell signaling pathways that promote inflammation and tissue injury (Figure 3).2

Figure 3. Hypotheses of sulphur mustard toxicity. Cytotoxicity is induced through pathways involving (1) PARP (green) and (2) the inactivation of glutathione (blue). Alkylation of target molecules generates reactive oxygen species, principally the superoxide anion (O2-.) and the highly toxic and reactive hydroxyl radical (OH.), which can induce lipid peroxidation of cell membranes. Reduced glutathione (GSH) acts as a substrate in the glutathione peroxidase (GPx) reaction producing oxidized glutathione (GSSG), which is recycled back to its reduced form by glutathione reductase (GSR) at the expense of NADPH. Depletion of GSH removes protection from these oxidants.
One hypothesis proposes that DNA strand breaks activate the DNA repair enzyme poly (ADP-ribose) polymerase (PARP),3 which depletes its cofactor and substrate nicotinic adenine dinucleotide (NAD), impairing glycolysis and ATP production and leading to cell death. Subsequent release of proteases from keratinocytes degrades basement membrane proteins disrupting the epidermal-dermal junction.4 PARP also activates nitric oxide synthase (NOS), which converts L-arginine (L-Arg) to the nitric oxide radical (NO.). NO. then reacts with superoxide (O2.-) to form the peroxynitrite radical (ONOO.-) further activating PARP and exacerbating genomic damage.5
SM also interacts with the free radical scavenger glutathione (GSH), which leads to the accumulation of endogenous oxidants and lipid peroxidation of cell membranes. Additionally, SM impairs glutathione reductase (GSR), increasing intracellular GSSH levels and further depleting GSH. Low cellular stores of GSH inactivate thiol proteins, including calcium and magnesium triphosphatases that regulate calcium levels. Toxic increases in cytosolic calcium stimulate the production of proteases, endonucleases, and phospholipases that break down cytoskeleton, DNA, and cell membranes, respectively (Figure 3).6
Oxidative stress also triggers the NF-κB pathway through various receptors (eg, RANK, TNFR, TLR) that activate catalytic IκB kinase 2, initiating serine-phosphorylation and ubiquitination. The ensuing proteasomal degradation of IκBα liberates NF-κB, allowing it to translocate to the nucleus and activate signaling pathways and gene expression that upregulates proinflammatory cytokines (eg, interleukin -1 [IL-1], IL-8, Il-6, and TNF-α). This results in inflammation or apoptosis.7
Q2. What are the clinical features of sulfur mustard injury?
Exposure to SM targets the skin, eyes, and airways but can also affect the gastrointestinal tract and cause marrow depression with secondary infections and death. The severity of clinical manifestations is dose-dependent, with early symptoms comprising conjunctivitis, photophobia, lacrimation, cough, hoarseness, sore throat, and edema of the eyelids.8
Within 4 hours, erythema is accompanied by itching and burning with a predilection for moist areas, such as the antecubital fossae, perineum, external genitalia, and axillae (Figure 2E). Vesicles emerge and coalesce to form pendulous blisters and slough, discharging a nontoxic amber serous fluid, leaving painful shallow ulcers with a raw moist bed, and resembling a superficial second-degree burn. Healing is slow and there is an increased risk of secondary infection. A pattern of inflammatory hyperpigmentation followed by blotchy desquamation of the epidermis and hypopigmentation was observed in the Iranian casualties (Figure 2B).8
High doses of SM can cause skin necrosis with absent blistering or doughnut blisters that surround a central necrotic zone. The agent is particularly toxic to the eyes, causing, keratitis, corneal erosion, perforation, and glaucoma.9 In severe respiratory exposure, sloughing of the epithelium may lead to respiratory obstruction with death from hemorrhagic pulmonary edema or bronchopneumonia and sepsis. Cholinergic toxicity can cause excessive salivation, cramping, diarrhea, vomiting, and constricted pupils.
Although the mortality rate from exposure to SM is low (2%-3% among Iranian casualties),10 the potential for a fatal outcome is predicted by rapid onset of respiratory symptoms, deep involvement of the skin affecting over 50% of the body, neurological symptoms such as muscle spasms and convulsions, and bone marrow involvement. The effect on the hematopoietic system is also related to the dose. Willems reported that 50% of the Iranian casualties reviewed had leukopenia. Among these, 7 died, 2 of whom had total marrow aplasia.8
Q3. How are casualties of sulfur mustard poisoning treated?
Prevention of exposure is provided by personal protective equipment, including butyl rubber gloves, boots, and a respirator. Studies in animal models indicate agents with the potential to prevent SM-induced inflammation, including topical anti-inflammatory medication, protease inhibitors, intracellular scavengers, cell cycle inhibitors, PARP inhibitors, calcium modulators, and antioxidants.11
Exposure demands immediate decontamination of clothing using Fuller’s earth and washing affected areas with water or preferably 0.5% sodium hypochlorite solution. The eyes should be irrigated with saline or water. For patients presenting with erythema and minor blistering, symptomatic relief may be achieved by topical application of calamine lotion, steroids, antihistamines, and nonsteroidal analgesics.
Patients with extensive burns warrant referral to a burn center where fluid replacement is tailored to hemodynamic parameters rather than the size of the burn.8 Large blisters are easily ruptured and are best debrided whereas those less than 1 cm in diameter should be left intact.
Application of an antibiotic ointment, such as silver sulfadiazine, and a petrolatum gauze dressing aids wound healing, with the added potential benefit of platelet-derived growth factor, keratinocyte-derived growth factor, and negative pressure wound therapy. Pulsed CO2 and Er: YAG laser ablation, powered dermabrasion, and enzymatic debridement with Debridase have been shown to accelerate wound healing in the pig model. For deep cutaneous injuries, superior results were achieved with tangential excision and the application of split-thickness skin grafts.12
Patients with eye involvement need ophthalmic consultation, daily irrigation, and topical antibiotic application 3 to 4 times per day. A mydriatic, such as atropine or homatropine, should also be used to keep the pupil dilated to prevent the formation of synechiae. Bronchospasm refractory to standard therapy may benefit from systemic steroids. For high-dose pulmonary exposure, early ventilatory support, bronchoscopy for pseudomembrane removal, and targeted antibiotic therapy are essential. Finally, bone marrow suppression requires isolation, transfusion, antibiotics, and the use of marrow stimulants.
Q4. What are the long-term effects of sulfur mustard exposure?
The long-term effects of SM on critical cellular macromolecules can lead to latent disease years later. A study conducted on 34000 Iranian casualties revealed that chronic skin, eye, and respiratory lesions were observed in 24.5%, 39.2%, and 42.5% of cases, respectively.13
Changes in the genomic architecture of cells have been found to include epigenetic alterations, such as DNA methylation and histone modifications that are thought to produce acute toxicity and chronic health outcomes such as cancer, immune dysfunction, and neurological disorders.14 Delayed dermatological complications included chronic dryness, pruritis, hair loss, skin rashes, fragile skin, actinic keratosis, and multiple basal cell carcinomas. Destruction of melanocytes can leave permanent hypopigmented areas of skin, although hyperpigmentation more commonly occurs (Figure 2B). Excessive wound contraction can cause disfigurement and restricted joint motion. The relationship between SM and cancer is debated, but severe exposure can cause chronic skin ulceration with the potential to undergo malignant transformation.12 Although estimates of lifetime cancer risk are not high, young children, with higher rates of cell division, may be more vulnerable to the carcinogenic potential of mutated cells.
Chronic conjunctivitis and corneal clouding may also accompany a delayed keratopathy that presents with recurrent or persistent corneal ulceration. Respiratory ailments include chronic bronchitis, asthma, recurrent pneumonia, bronchiectasis, tracheobronchial stenosis, and lung cancer.10 Late psychological complications revealed a significant incidence of depression, psychosis, and personality and post-traumatic stress disorders.15
Acknowledgments
Author: Stephen M. Milner, MBBS, BDS, DSc (Hon), FRCSE, FACS
Affiliation: Professor of Plastic and Reconstructive Surgery, Johns Hopkins University School of Medicine, Baltimore, MD (Ret.)
Correspondence: Stephen M. Milner, MBBS, BDS, DSc (Hon), FRCSE, FACS; stephenmilner123@gmail.com
Disclosures: The author discloses no relevant conflict of interest or financial disclosures for this manuscript.
References
1. Khateri S, Ghanei M, Soroush M, Haines D. Effects of mustard gas exposure in pediatric patients: Long−term health status of mustard−exposed children, 14 years after chemical bombardment of Sardasht. J Burns & Surg Wound Care. 2003;2(11).
2. Hurst CG, Petrali JP, Barillo DJ, et al. Vesicants. In: Lenhart MK, Tuorinsky SD, eds. Textbooks of Military Medicine. Medical Aspects of Chemical Warfare. Office of the Surgeon General at TMM Publications; 2008:259-310.
3. Papirmeister B, Feister AJ, Robinson SI, et al. Molecular Mechanisms of Cytotoxicity. In: Medical Defense against Mustard Gas: Toxic Mechanisms and Pharmacological Implications. CRC Press; 1991.
4. Jin X, Ray R, Ray P. Sulfur mustard-stimulated proteases and their inhibitors in a cultured normal human epidermal keratinocytes model: A potential approach for anti-vesicant drug development. Toxicol Rep. 2016;3:393-400. doi:10.1016/j.toxrep.2016.03.007
5. Parihar A, Parihar MS, Milner S, Bhat S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns. 2008;34(1):6-17. doi:10.1016/j.burns.2007.04.009
6. Orrenius S, Nicotera P. On the role of calcium in chemical toxicity. Arch Toxicol. 1987;11(Suppl):11-19.
7. Kehe K, Balszuweit F, Steinritz D, Thiermann H. Molecular toxicology of sulfur mustard-induced cutaneous inflammation and blistering. Toxicology. 2009;263(1):12-9. doi:10.1016/j.tox.2009.01.019
8. Willems J. Clinical management of mustard gas casualties. Ann Belg Med Mil. 1989;3(Suppl):1-61.
9. Panahi Y, Roshandel D, Sadoughi MM, Ghanei M, Sahebkar A. Sulfur mustard-induced ocular injuries: update on mechanisms and management. Curr Pharm Des. 2017;23(11):1589-1597. doi:10.2174/1381612822666161021150125
10. Mansour Razavi S, Salamati P, Saghafinia M, Abdollahi M. A review on delayed toxic effects of sulfur mustard in Iranian veterans. Daru. 2012;20(1):51. Published 2012 Oct 9. doi:10.1186/2008-2231-20-51
11. Casillas RP, Kiser RC, Truxall JA, Singer AW, Shumaker SM, Niemuth NA, Ricketts KM, Mitcheltree LW, Castrejon LR, Blank JA. Therapeutic approaches to dermatotoxicity by sulfur mustard. I. Modulaton of sulfur mustard-induced cutaneous injury in the mouse ear vesicant model. J Appl Toxicol. 2000;20 (Suppl 1): S145-151. doi:10.1002/1099-1263(200012)20:1+<::aid-jat665>3.0.co;2-j
12. Graham JS, Chilcott RP, Rice P, Milner SM, Hurst CG, Maliner BI. Wound healing of cutaneous sulfur mustard injuries: strategies for the development of improved therapies. J Burns Wounds. 2005;4:e1.
13. Khateri S, Ghanei M, Keshavarz S, Soroush M, Haines D. Incidence of lung, eye, and skin lesions as late complications in 34,000 Iranians with wartime exposure to mustard agent. J Occup Environ Med. 2003;45(11):1136-1143. doi:10.1097/01.jom.0000094993.20914.d1
14. Simons T, Steinritz D, Bölck B, Schmidt A, Popp T, Thiermann H, Gudermann T, Bloch W, Kehe K. Sulfur mustard-induced epigenetic modifications over time - a pilot study. Toxicol Lett. 2018;293:45-50. doi:10.1016/j.toxlet.2017.11.010
15. Roshan R, Rahnama P, Ghazanfari Z, et al. Long-term effects of sulfur mustard on civilians’ mental health 20 years after exposure (The Sardasht-Iran Cohort Study). Health Qual Life Outcomes. 2013;11:69. doi:10.1186/1477-7525-11-69