Pathogenesis of Pressure Injuries
© 2024 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of ePlasty or HMP Global, their employees, and affiliates.
Questions
1. How are pressure injuries initiated?
2. What is the role of ischemia in the pathogenesis of pressure injuries?
3. What is the significance of the wound microbiome?
4. Does spinal cord injury affect healing of a chronic wound?
Case Description
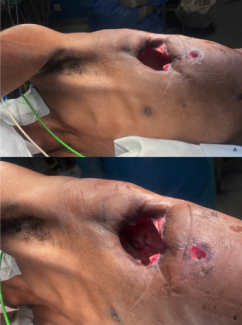
A 30-year-old paraplegic male presented with a chronic draining wound overlying his left ischial tuberosity 5 years after sustaining a gunshot wound to his spine at the level of T6.

Q1. How are pressure injuries initiated?
Deep pressure injuries (PIs) occur following severe or sustained mechanical loading of tissue, which causes deformation of cells, inflammation, and ischemia. The injury progresses from a microscopic to a macroscopic level.1 How cells react to stress is vital to understanding the pathophysiology of PIs. There is no sudden transition from normal to necrotic tissue. In any PI there is a zone of healthy tissue, an injured zone that is reversible, and an irreparable zone.2 Because different tissues vary in their susceptibility to stress, and muscle is the most sensitive, deep PIs originate in the soft tissues adjacent to bony prominences and progress, often unnoticeably, toward the intact surface of the skin (Figure 1).
The integrity of the plasma membrane is protected by the actin cytoskeleton. Mechanical forces can fragment the actin, leading to irreversible deformation of the cell membrane, membrane leakage, and cell rupture. Stress can also be transmitted to the nucleus3 to modify gene expression, which causes apoptosis.
Q2. What is the role of ischemia in the pathogenesis of pressure injuries?
When the pressure at the interface between skin and supporting surfaces exceeds capillary closing pressure (32 mm Hg), the tissues are deprived of oxygen and nutrients.4 Hypoxia impairs oxidative phosphorylation in the mitochondrial electron transport system, depleting adenosine triphosphate (ATP). Compensatory anaerobic metabolism creates insufficient ATP production to fuel ATPase-dependent transport systems (eg, Na+/K+ ATPase, Ca2+ ATPase). To buffer lactic acidosis, H+ ions are exchanged for Na+ ions, which are in turn exchanged for Ca2+ ions.5 Accumulation of Na+ causes osmotic cell swelling and rupture, and excessive intracellular calcium flux activates phospholipases, proteases, and endonucleases that fragment membranes, cytoskeletal proteins, and nucleic acids, respectively (Figure 2).


Restoration of blood flow to ischemic tissues, as may occur in patients subject to turning regimens, produces toxic reactive species formed mainly by the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase system, nitric oxide synthase system, xanthine oxidase system, and polyadenosine-diphosphate-ribose polymerase (PARP-1) activation,6 processes enhanced by an imbalance between oxidant and antioxidant enzyme systems.7 Generation of the highly reactive hydroxyl (OH*) radical induces lipid peroxidation of cell and organelle membranes as well as macromolecules (Figure 3).
Necrosis is augmented by leakage of hydrolytic enzymes from lysosomes. Calcium overload and ATP depletion are important in proapoptotic signaling. This favors opening of the mitochondrial permeability transition pore (mPTP) and leakage of cytochrome C, which triggers the caspase cascade culminating in cycles of apoptosis as a common end-effector of ischemia/reperfusion-induced cell lysis and death.8 Apoptosis is also induced extrinsically by an interaction between a death receptor on the cell surface (eg, TNFR-1) and its ligand (eg, FasL) expressed on cytotoxic T-cells and by endoplasmic reticulum (ER) stress, which increases protein misfolding.
Oxidants also increase expression of surface endothelial adhesion molecules. This results in excessive recruitment of neutrophils and release of proinflammatory cytokines, oxidants, and metalloproteases, which degrade growth factors, their receptors, and extracellular matrix to maintain the wound in a state of chronic inflammation.
Q3. What is the significance of the wound microbiome?
Common invasive species of chronic wounds include Pseudomonas aeruginosa, Staphylococcus aureus, beta-hemolytic streptococci, and Clostridium. Landmark studies have defined clinical wound infection as those with colony counts >105 per gram of tissue as determined by tissue biopsy and quantitative culture.9 Unfortunately, quantitative culture of wounds is not routinely practiced, and DNA sequencing techniques reveal that standard culture underestimates bacterial diversity.10 Moreover, it seems likely that virulence will vary with the bacterial species and with multiple organisms. Any number of alpha-hemolytic streptococci are considered detrimental.11
The majority of bacteria, however, do not exist as planktonic organisms but as biofilms. These are surface-adhering bacteria protected by a glycocalyx that increases resistance to antibodies, phagocytic cells, and antimicrobial agents. The microenvironment of necrotic tissue, low oxygen tension, and diminished host immune response encourages proliferation of bacteria in the wound. An influx of neutrophils elevates the level of proinflammatory cytokines, which increase production of matrix metalloproteases (MMPs) and decrease the level of tissue inhibitors of MMPs.12 The result is a degradation of the extracellular matrix, decreased production of growth factors, inhibition of phagocytosis by impeding complement-activated opsonization of bacteria, and attenuation of the neutrophil oxidative burst.
MMPs are also believed to be chemotactic for neutrophils so that this cycle can only be broken by decreasing the bacterial count. This is best achieved by debridement (sharp, mechanical, or enzymatic) as well as drainage of abscesses and removal of infected bone and nonviable tissue, slough, and biofilm. It allows removal of callus and necrotic tissue, reduction in bacterial burden, and removal of abnormal wound edge tissue.
Q4. Does spinal cord injury affect wound healing?
Paraplegic patients also suffer impaired wound healing. The peripheral nervous system and central nervous system can modulate all phases of wound healing through the secretion of neuropeptides (Figure 4). Below the level of injury, deficiency of these neuromediators can hinder angiogenesis, keratinocyte and fibroblast proliferation, collagen production, and remodeling. Tissue repair may be further impaired by interruption of vasomotor pathways that cause hypotension and reduce oxygen delivery to the tissues. Peripheral ischemic processes have been shown to upregulate neuropeptides in the nervous systems, and spinal cord injury may remove this adaptive response to hypoxia.13,14

BDNF, brain-derived neurotrophic factor; CGRP, calcitonin gene-related peptide; CRH, corticotropin-releasing hormone; SP, substance P; NPY, neuropeptide Y; Gal, galanoin; GRP, gastrin-releasing peptide; NGF, nerve growth factor; NKA, neurokinin A; NT-3, neurotrophin-3; PACAP, pituitary adenylate cyclase-activating peptide; VIP, vasoactive intestinal.
Acknowledgments
Affiliations: 1Professor of Plastic and Reconstructive Surgery, Johns Hopkins University School of Medicine, Baltimore, Maryland (Ret.); 2 Department of Research and Development, PolarityTE, Inc, Salt Lake City, Utah
Correspondence: Stephen M. Milner, MBBS, BDS, DSc (Hon) FRCSE, FACS;stephenmilner123@gmail.com
Disclosures: The authors disclose no financial or other conflicts of interest.
References
1. Gefen A, Weihs D. Cytoskeleton and plasma-membrane damage resulting from exposure to sustained deformations: A review of the mechanobiology of chronic wounds. Med Eng Phys. 2016;38(9):828-833. doi:10.1016/j.medengphy.2016.05.014
2. Smit JH. Four ways cells may die. LinkedIn. Published 2017. https://www.linkedin.com/pulse/4-ways-cell-may-die-harm-jaap-smit/
3. Belaadi N, Aureille J, Guilluy C. Under pressure: Mechanical stress management in the nucleus. Cells. 2016;5(2):1-11. doi:10.3390/cells5020027
4. Gawlitta D, Li W, Oomens CWJ, Baaijens FPT, Bader DL, Bouten CVC. The relative contributions of compression and hypoxia to development of muscle tissue damage: An in vitro study. Ann Biomed Eng. 2007;35(2):273-284. doi:10.1007/s10439-006-9222-5
5. Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Ischemia/reperfusion. Compr Physiol. 2017;7(1):113-170. doi:10.1002/cphy.c160006
6. Parihar A, Parihar MS, Milner S, Bhat S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns. 2008;34(1):6-17. doi:10.1016/j.burns.2007.04.009
7. Wu MY, Yiang GT, Liao WT, et al. Current mechanistic concepts in ischemia and Reperfusion injury. Cell Physiol Biochem. 2018;46(4):1650-1667. doi:10.1159/000489241
8. Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in chronic wounds. Int J Mol Sci. 2016;17(12). doi:10.3390/ijms17122085
9. Robson MC. A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am . 1997;77(3):637-650. doi:10.1016/s0039-6109(05)70572-7
10. Rhoads DD, Wolcott RD, Sun Y, Dowd SE. Comparison of culture and molecular identification of bacteria in chronic wounds. Int J Mol Sci. 2012;13(3):2535-2550. doi:10.3390/ijms13032535
11. Metcalf D, Bowler P. Biofilm delays wound healing: A review of the evidence. Burns Trauma. 2013;1(1):5. doi:10.4103/2321-3868.113329
12. Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen. 1996;4(3):321-325. doi:10.1046/j.1524-475X.1996.40307.x
13. Gherardini G, Evans GRD, Theodorsson E, et al. Calcitonin gene-related peptide in experimental ischemia. Implication of an endogenous anti-ischemic effect. Ann Plast Surg. 1996;36(6):616-620. doi:10.1097/00000637-199606000-00009
14. Ashrafi M, Baguneid M, Bayat A. The role of neuromediators and innervation in cutaneous wound healing. Acta Derm Venereol. 2016;96(5):587-597. doi:10.2340/00015555-2321