 A 53-year-old otherwise healthy man was referred to us by his primary care provider for the evaluation of a large, asymptomatic, polypoid mass over his left lateral leg that he had for more than 25 years (Figure 1). He could not recall exactly how this lesion started, but said that it began as a very small bump similar to 2 other bumps on his legs. However, the lesion slowly grew, and about 15 years ago he traumatized it against a ledge, after which the lesion bled and “fell off.” It then began to grow again at a very slow rate and was completely asymptomatic. On examination, there was a 4.0 x 3.5 cm nontender, pink, firm, smooth surfaced, pedunculate, and exophytic mass over the left lateral leg. The lesion was clinically well circumscribed with a slightly indurated base.
A 53-year-old otherwise healthy man was referred to us by his primary care provider for the evaluation of a large, asymptomatic, polypoid mass over his left lateral leg that he had for more than 25 years (Figure 1). He could not recall exactly how this lesion started, but said that it began as a very small bump similar to 2 other bumps on his legs. However, the lesion slowly grew, and about 15 years ago he traumatized it against a ledge, after which the lesion bled and “fell off.” It then began to grow again at a very slow rate and was completely asymptomatic. On examination, there was a 4.0 x 3.5 cm nontender, pink, firm, smooth surfaced, pedunculate, and exophytic mass over the left lateral leg. The lesion was clinically well circumscribed with a slightly indurated base.
What is your diagnosis?
To learn the answer, go to page 2
{{pagebreak}}
Diagnosis: Giant Dermatofibroma
Dermatofibroma (DF) is a common benign cutaneous lesion that occurs most frequently on the lower legs of women. Several clinical and pathological subtypes of DF have been described. Giant DF is a rare and unusual clinical variant that often presents as an exophytic or pedunculated mass.1 The large size and sometimes fungating appearance of these lesions may raise suspicion for malignancy. However, these lesions are histologically and biologically benign.
Several clinical and histologic variants of DF have been described, with many lesions containing combined features. Clinical variants include common DF, multiple DFs, eruptive DFs, atrophic DF, atypical polypoid DF, and giant DF.2 Multiple and/or eruptive DFs, either clustered or widespread, can be seen in autoimmune disorders (systemic lupus erythematosus, myasthenia gravis), in the setting of immunosuppression (HIV, infection), in atopic dermatitis, in pregnancy, as well as in a familial pattern.2,3 The other clinical subtypes are exceedingly rare, with limited case reports in the literature. Histologic variants are numerous and outlined in the Table. The histologic features of several variants may be found within the same lesion.4
Giant DF is a rare clinical variant of DF characterized by unusually large size, pedunculated appearance, benign behavior, and histopathologic features identical to that of common DFs. Although there is no clear definition of the minimal size required for the diagnosis of giant DF, several authors have used ≥3.5 cm to describe these lesions.2,5
The first case was reported in 1975 by Danckeart and Karassic as a 3.5 x 3.5 cm lesion on the pretibial area of a 52-year-old man. To date, 27 giant DFs have been reported, ranging from 3.5 to 30 cm.1,6,7
Clinical Presentations
DF usually presents as a slow-growing, small (≤1 cm), solitary, firm, and well-circumscribed dermal nodule with a brown or reddish-brown hue (Figure 1). The lesion often induces slight inward retraction of the overlying skin, and lateral compression may further exhibit this feature, creating a “dimple sign,” which indicates tethering of the overlying epidermis to the lesion. DFs are typically found on the legs but may also occur on various other parts of the body, and are more common in middle-aged women. They are usually asymptomatic, but can sometimes be pruritic or tender.
Although common DFs are easily recognized using only visual inspection and palpation, dermoscopy can help confirm the diagnosis. The classic finding under dermoscopy is a central white scar-like patch surrounded by a peripheral rim of hyperpigmentation.
Giant DF presents as a firm, exophytic, and often polypoid mass that may ulcerate or bleed. The surface may be smooth or keratotic. Purulent drainage, plaque-like presentation, and satellite lesions have also been reported features.8 Almost all of the cases have occurred on the legs, but they have also been reported in the groin,9 on the lower back of a pregnant woman,10 and on the posterior shoulder.11 Growth may be rapid or slow.9,10
Pathogenesis
The etiology of DFs is controversial. Many authors believe that they occur as a reactive process following some type of insult such as arthropod bite, trauma, folliculitis, or an inflammatory condition. Others consider DFs to be neoplastic processes due to the persistent nature of these lesions, as well as clonality of the proliferating cells.12 However, clonality has also been observed in various inflammatory conditions such as atopic dermatitis, psoriasis, and lichen sclerosis, thus this feature alone does not necessarily represent a neoplastic process.4
Whether DFs are reactive or neoplastic is still widely debated, however, most consider DFs to be reactive processes. The pathogenesis of giant DFs is even more obscure and only speculative.
 Histopathology
Histopathology
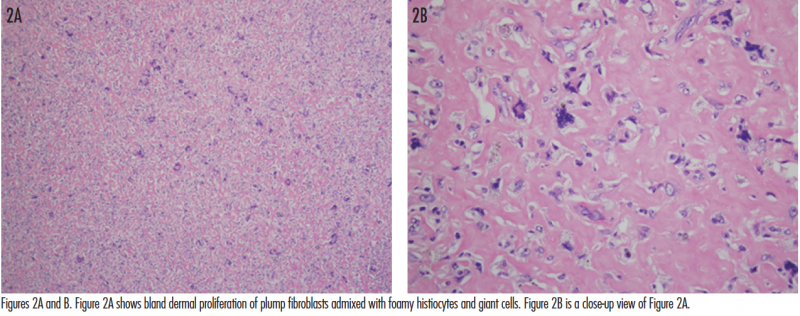
The histopathologic findings of giant DFs are fairly identical to those of common DFs. Typically, there is a fairly well-circumscribed dermal proliferation with aggregates of spindle-shaped fibroblasts arranged between thickened collagen bundles. The fibroblasts have plump nuclei, and although mitoses may be present, they are usually not numerous. Foamy histiocytes may be seen in variable numbers, and sometimes form multinucleated giant cells. There may be peripheral collagen trapping and an increased number of small blood vessels. DFs can become hemorrhagic with subsequent formation of hemosiderin deposits. The overlying epidermis is hyperplastic with hyperpigmentation of the basal layer. Sometimes follicular induction may be observed, where basaloid-appearing extensions emanate from the overlying epidermis and may mimic basal cell carcinoma (BCC).13 However, there have been some reports in the literature of BCC overlying a DF. Rosmaninho and colleagues7 reported a case where well-developed basaloid nests were seen extending from the epidermis and overlying a DF. They observed retraction artifact around the basaloid structures, positive Ber-EP4 staining, and even focal infiltration into the reticular dermis. This led to the diagnosis of BCC overlying a DF, and excision was performed. The differentiation between true BCC vs follicular induction overlying DFs remains a controversial topic.
Probably due to their large size, giant DFs may also have additional histologic features such as ulceration, areas of cystic degeneration, focal hemorrhage, and cholesterol clefting.4,9 It is especially important to distinguish giant DF from dermatofibrosarcoma protuberans (DFSP) histologically, and immunohistochemical markers are often helpful. DF is usually strongly positive for factor XIIIa, and negative for CD34. In contrast, factor XIIIa tends to be negative in DFSP, and CD34 tends to be positive. In one case, histopathologic examination demonstrated most conventional features of DF; however, there were some architectural features of DFSP. Staining with factor XIIIa and CD34 solidified the diagnosis of giant DF in that case.5 Despite the utility of these markers, there may still be variations in staining among different cases. Some DFs demonstrate focal CD34 reactivity, whereas some DFSPs paradoxically express factor XIIIa but not CD34.14 In such cases, stromelysin 3 (ST3) staining may be useful. Cribier and colleagues14 reported that of 40 cases of DFSP, none expressed ST3, while all 40 cases of DF including 10 giant DFs expressed ST3. In terms of architecture, Kamino and Jacobson15 described how to differentiate DF extending into the fat from DFSP, and found that DF primarily extends in an irregular fashion with a vertical or radial pattern, predominantly along the septa. They concluded that DF and DFSP had different patterns of fat extension. Thus, the architecture of the lesion along with characteristic immunostaining patterns help to differentiate DF from DFSP.
Differential Diagnosis
Given the large tumor-like appearance of giant DF, it can often be mistaken for malignant lesions. The most important entity in the differential diagnosis is DFSP. Other lesions that may present similarly include sarcoma, BCC, squamous cell carcinoma, melanoma, eccrine acrospiroma, fibroma, pyogenic granuloma, keloid, malignant fibrous histiocytoma, and collagenoma.
Treatment and Prognosis
Giant DF has a benign course, and surgical excision is curative. However, removal of the lesion is not necessary unless there is diagnostic uncertainty or patient discomfort.4 To date, recurrence after surgical excision has not been reported, even after incomplete excision.2,4,6
Our Patient
A shave removal of the lesion was performed with a deep-shave technique. The base was then electrodesiccated. Microscopic examination of the lesion revealed a bland spindle cell proliferation consistent with DF (Figures 2A and B). At 2-week follow-up, the wound was healing well. The patient was informed of the benign etiology of the mass, and that complete excision was an option but not a requirement. With the understanding that there is a possibility of local recurrence, the patient deferred excision at this time. He will be monitored periodically for any evidence of recurrence.
Conclusion
 Giant DF is a rare clinical variant of DF characterized by its unusually large size, with fewer than 30 cases reported to date. It can often be misdiagnosed as a malignancy due to its striking appearance. However, clinicians should be aware that giant DF is a benign process and that surgical excision, though not required, is curative. To our knowledge, recurrence after excision of a giant DF has not been reported, nor have there been reports of diagnosis revision to DFSP after re-excision. However, the possibility of missing the diagnosis of DFSP with a superficial biopsy still remains a valid concern for dermatologists and dermatologic surgeons.
Giant DF is a rare clinical variant of DF characterized by its unusually large size, with fewer than 30 cases reported to date. It can often be misdiagnosed as a malignancy due to its striking appearance. However, clinicians should be aware that giant DF is a benign process and that surgical excision, though not required, is curative. To our knowledge, recurrence after excision of a giant DF has not been reported, nor have there been reports of diagnosis revision to DFSP after re-excision. However, the possibility of missing the diagnosis of DFSP with a superficial biopsy still remains a valid concern for dermatologists and dermatologic surgeons.
 Dr Liu is with the department of dermatology at the State University of New York Downstate Medical Center in Brooklyn, NY.
Dr Liu is with the department of dermatology at the State University of New York Downstate Medical Center in Brooklyn, NY.
Dr Alapati is with the department of dermatology at Veteran Affairs Medical Center in Brooklyn, NY.
Dr Khachemoune, the Section Editor of Derm DX, is with the department of dermatology at Veteran Affairs Medical Center, and the department of dermatology at the State University of New York Downstate, both in Brooklyn, NY.
Disclosure: The authors report no relevant financial relationships.
References
1. Danckaert KB, Karassik SL. Dermatofibroma: an unusual presentation. Cutis. 1975;16:245-247.
2. Requena L, Fariña MC, Fuente C, et al. Giant dermatofibroma. A little-known clinical variant of dermatofibroma. J Am Acad Dermatol. 1994;30 (5 Pt 1):714-718.
3. Niiyama S, Katsuoka K, Happle R, Hoffmann R. Multiple eruptive dermatofibromas: a review of the literature. Acta Derm Venereol. 2002;82(4):241-244.
4. Pusztaszeri M, Jaquet PY, Williamson C. Giant hemosiderotic dermatofibroma: a case report and review of the literature. Case Rep Dermatol. 2011;18;3(1):32-36.
5. Hoshina D, Shibaki A, Aoyagi S, Kimura K, Shimizu H. Giant dermatofibroma: a rare variant of dermatofibroma preferentially developing on the lower limbs. Clin Exp Dermatol. 2007;32(1):132-134.
6. Kalsi H, Rahman A, Harbol T, Sidhu J. Giant hemosiderotic dermatofibroma: the largest giant dermatofibroma reported to date. Am J Dermatopathol. 2015;37(10):778-782.
7. Rosmaninho A, Farrajota P, Peixoto C, Amorim I, Selores M. Basal cell carcinoma overlying a dermatofibroma: a revisited controversy. Eur J Dermatol. 2011;21(1):137-138.
8. Sehgal VN, Sardana K, Khandpur S, Sharma S, Majumdar S, Aggarwal AK. Giant combined dermatofibroma with satellitosis. Clin Exp Dermatol. 2004;29(2):147-149.
9. Lang KJ, Lidder S, Hofer M, Graham C, Taylor A. Rapidly evolving giant dermatofibroma. Case Rep Med. 2010;2010:620910.
10. Micantonio T, Fargnoli MC, Peris K. Giant dermatofibroma appearing during pregnancy. Acta Derm Venereol. 2006;86(1):86-87.
11. Hueso L, Sanmartín O, Alfaro-Rubio A, et al. Giant dermatofibroma: case report and review of the literature. Actas Dermosifiliogr. 2007;98(2):121-124.
12. Chen TC, Kuo T, Chan HL. Dermatofibroma is a clonal proliferative disease. J Cutan Pathol. 2000;27(1):36-39.
13. Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. St. Louis, MO: W.B. Saunders; 2012:1962-1964.
14. Cribier B, Noacco G, Peltre B, Grosshans E. Stromelysin 3 expression: a useful marker for the differential diagnosis dermatofibroma versus dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2002;46(3):408-413.
15. Kamino H, Jacobson M. Dermatofibroma extending into the subcutaneous tissue. Differential diagnosis from dermatofibrosarcoma protuberans. Am J Surg Pathol. 1990;14(12):1156-1164.
 A 53-year-old otherwise healthy man was referred to us by his primary care provider for the evaluation of a large, asymptomatic, polypoid mass over his left lateral leg that he had for more than 25 years (Figure 1). He could not recall exactly how this lesion started, but said that it began as a very small bump similar to 2 other bumps on his legs. However, the lesion slowly grew, and about 15 years ago he traumatized it against a ledge, after which the lesion bled and “fell off.” It then began to grow again at a very slow rate and was completely asymptomatic. On examination, there was a 4.0 x 3.5 cm nontender, pink, firm, smooth surfaced, pedunculate, and exophytic mass over the left lateral leg. The lesion was clinically well circumscribed with a slightly indurated base.
A 53-year-old otherwise healthy man was referred to us by his primary care provider for the evaluation of a large, asymptomatic, polypoid mass over his left lateral leg that he had for more than 25 years (Figure 1). He could not recall exactly how this lesion started, but said that it began as a very small bump similar to 2 other bumps on his legs. However, the lesion slowly grew, and about 15 years ago he traumatized it against a ledge, after which the lesion bled and “fell off.” It then began to grow again at a very slow rate and was completely asymptomatic. On examination, there was a 4.0 x 3.5 cm nontender, pink, firm, smooth surfaced, pedunculate, and exophytic mass over the left lateral leg. The lesion was clinically well circumscribed with a slightly indurated base.
What is your diagnosis?
Diagnosis: Giant Dermatofibroma
Dermatofibroma (DF) is a common benign cutaneous lesion that occurs most frequently on the lower legs of women. Several clinical and pathological subtypes of DF have been described. Giant DF is a rare and unusual clinical variant that often presents as an exophytic or pedunculated mass.1 The large size and sometimes fungating appearance of these lesions may raise suspicion for malignancy. However, these lesions are histologically and biologically benign.
Several clinical and histologic variants of DF have been described, with many lesions containing combined features. Clinical variants include common DF, multiple DFs, eruptive DFs, atrophic DF, atypical polypoid DF, and giant DF.2 Multiple and/or eruptive DFs, either clustered or widespread, can be seen in autoimmune disorders (systemic lupus erythematosus, myasthenia gravis), in the setting of immunosuppression (HIV, infection), in atopic dermatitis, in pregnancy, as well as in a familial pattern.2,3 The other clinical subtypes are exceedingly rare, with limited case reports in the literature. Histologic variants are numerous and outlined in the Table. The histologic features of several variants may be found within the same lesion.4
Giant DF is a rare clinical variant of DF characterized by unusually large size, pedunculated appearance, benign behavior, and histopathologic features identical to that of common DFs. Although there is no clear definition of the minimal size required for the diagnosis of giant DF, several authors have used ≥3.5 cm to describe these lesions.2,5
The first case was reported in 1975 by Danckeart and Karassic as a 3.5 x 3.5 cm lesion on the pretibial area of a 52-year-old man. To date, 27 giant DFs have been reported, ranging from 3.5 to 30 cm.1,6,7
Clinical Presentations
DF usually presents as a slow-growing, small (≤1 cm), solitary, firm, and well-circumscribed dermal nodule with a brown or reddish-brown hue (Figure 1). The lesion often induces slight inward retraction of the overlying skin, and lateral compression may further exhibit this feature, creating a “dimple sign,” which indicates tethering of the overlying epidermis to the lesion. DFs are typically found on the legs but may also occur on various other parts of the body, and are more common in middle-aged women. They are usually asymptomatic, but can sometimes be pruritic or tender.
Although common DFs are easily recognized using only visual inspection and palpation, dermoscopy can help confirm the diagnosis. The classic finding under dermoscopy is a central white scar-like patch surrounded by a peripheral rim of hyperpigmentation.
Giant DF presents as a firm, exophytic, and often polypoid mass that may ulcerate or bleed. The surface may be smooth or keratotic. Purulent drainage, plaque-like presentation, and satellite lesions have also been reported features.8 Almost all of the cases have occurred on the legs, but they have also been reported in the groin,9 on the lower back of a pregnant woman,10 and on the posterior shoulder.11 Growth may be rapid or slow.9,10
Pathogenesis
The etiology of DFs is controversial. Many authors believe that they occur as a reactive process following some type of insult such as arthropod bite, trauma, folliculitis, or an inflammatory condition. Others consider DFs to be neoplastic processes due to the persistent nature of these lesions, as well as clonality of the proliferating cells.12 However, clonality has also been observed in various inflammatory conditions such as atopic dermatitis, psoriasis, and lichen sclerosis, thus this feature alone does not necessarily represent a neoplastic process.4
Whether DFs are reactive or neoplastic is still widely debated, however, most consider DFs to be reactive processes. The pathogenesis of giant DFs is even more obscure and only speculative.
 Histopathology
Histopathology
The histopathologic findings of giant DFs are fairly identical to those of common DFs. Typically, there is a fairly well-circumscribed dermal proliferation with aggregates of spindle-shaped fibroblasts arranged between thickened collagen bundles. The fibroblasts have plump nuclei, and although mitoses may be present, they are usually not numerous. Foamy histiocytes may be seen in variable numbers, and sometimes form multinucleated giant cells. There may be peripheral collagen trapping and an increased number of small blood vessels. DFs can become hemorrhagic with subsequent formation of hemosiderin deposits. The overlying epidermis is hyperplastic with hyperpigmentation of the basal layer. Sometimes follicular induction may be observed, where basaloid-appearing extensions emanate from the overlying epidermis and may mimic basal cell carcinoma (BCC).13 However, there have been some reports in the literature of BCC overlying a DF. Rosmaninho and colleagues7 reported a case where well-developed basaloid nests were seen extending from the epidermis and overlying a DF. They observed retraction artifact around the basaloid structures, positive Ber-EP4 staining, and even focal infiltration into the reticular dermis. This led to the diagnosis of BCC overlying a DF, and excision was performed. The differentiation between true BCC vs follicular induction overlying DFs remains a controversial topic.
Probably due to their large size, giant DFs may also have additional histologic features such as ulceration, areas of cystic degeneration, focal hemorrhage, and cholesterol clefting.4,9 It is especially important to distinguish giant DF from dermatofibrosarcoma protuberans (DFSP) histologically, and immunohistochemical markers are often helpful. DF is usually strongly positive for factor XIIIa, and negative for CD34. In contrast, factor XIIIa tends to be negative in DFSP, and CD34 tends to be positive. In one case, histopathologic examination demonstrated most conventional features of DF; however, there were some architectural features of DFSP. Staining with factor XIIIa and CD34 solidified the diagnosis of giant DF in that case.5 Despite the utility of these markers, there may still be variations in staining among different cases. Some DFs demonstrate focal CD34 reactivity, whereas some DFSPs paradoxically express factor XIIIa but not CD34.14 In such cases, stromelysin 3 (ST3) staining may be useful. Cribier and colleagues14 reported that of 40 cases of DFSP, none expressed ST3, while all 40 cases of DF including 10 giant DFs expressed ST3. In terms of architecture, Kamino and Jacobson15 described how to differentiate DF extending into the fat from DFSP, and found that DF primarily extends in an irregular fashion with a vertical or radial pattern, predominantly along the septa. They concluded that DF and DFSP had different patterns of fat extension. Thus, the architecture of the lesion along with characteristic immunostaining patterns help to differentiate DF from DFSP.
Differential Diagnosis
Given the large tumor-like appearance of giant DF, it can often be mistaken for malignant lesions. The most important entity in the differential diagnosis is DFSP. Other lesions that may present similarly include sarcoma, BCC, squamous cell carcinoma, melanoma, eccrine acrospiroma, fibroma, pyogenic granuloma, keloid, malignant fibrous histiocytoma, and collagenoma.
Treatment and Prognosis
Giant DF has a benign course, and surgical excision is curative. However, removal of the lesion is not necessary unless there is diagnostic uncertainty or patient discomfort.4 To date, recurrence after surgical excision has not been reported, even after incomplete excision.2,4,6
Our Patient
A shave removal of the lesion was performed with a deep-shave technique. The base was then electrodesiccated. Microscopic examination of the lesion revealed a bland spindle cell proliferation consistent with DF (Figures 2A and B). At 2-week follow-up, the wound was healing well. The patient was informed of the benign etiology of the mass, and that complete excision was an option but not a requirement. With the understanding that there is a possibility of local recurrence, the patient deferred excision at this time. He will be monitored periodically for any evidence of recurrence.
Conclusion
 Giant DF is a rare clinical variant of DF characterized by its unusually large size, with fewer than 30 cases reported to date. It can often be misdiagnosed as a malignancy due to its striking appearance. However, clinicians should be aware that giant DF is a benign process and that surgical excision, though not required, is curative. To our knowledge, recurrence after excision of a giant DF has not been reported, nor have there been reports of diagnosis revision to DFSP after re-excision. However, the possibility of missing the diagnosis of DFSP with a superficial biopsy still remains a valid concern for dermatologists and dermatologic surgeons.
Giant DF is a rare clinical variant of DF characterized by its unusually large size, with fewer than 30 cases reported to date. It can often be misdiagnosed as a malignancy due to its striking appearance. However, clinicians should be aware that giant DF is a benign process and that surgical excision, though not required, is curative. To our knowledge, recurrence after excision of a giant DF has not been reported, nor have there been reports of diagnosis revision to DFSP after re-excision. However, the possibility of missing the diagnosis of DFSP with a superficial biopsy still remains a valid concern for dermatologists and dermatologic surgeons.
 Dr Liu is with the department of dermatology at the State University of New York Downstate Medical Center in Brooklyn, NY.
Dr Liu is with the department of dermatology at the State University of New York Downstate Medical Center in Brooklyn, NY.
Dr Alapati is with the department of dermatology at Veteran Affairs Medical Center in Brooklyn, NY.
Dr Khachemoune, the Section Editor of Derm DX, is with the department of dermatology at Veteran Affairs Medical Center, and the department of dermatology at the State University of New York Downstate, both in Brooklyn, NY.
Disclosure: The authors report no relevant financial relationships.
References
1. Danckaert KB, Karassik SL. Dermatofibroma: an unusual presentation. Cutis. 1975;16:245-247.
2. Requena L, Fariña MC, Fuente C, et al. Giant dermatofibroma. A little-known clinical variant of dermatofibroma. J Am Acad Dermatol. 1994;30 (5 Pt 1):714-718.
3. Niiyama S, Katsuoka K, Happle R, Hoffmann R. Multiple eruptive dermatofibromas: a review of the literature. Acta Derm Venereol. 2002;82(4):241-244.
4. Pusztaszeri M, Jaquet PY, Williamson C. Giant hemosiderotic dermatofibroma: a case report and review of the literature. Case Rep Dermatol. 2011;18;3(1):32-36.
5. Hoshina D, Shibaki A, Aoyagi S, Kimura K, Shimizu H. Giant dermatofibroma: a rare variant of dermatofibroma preferentially developing on the lower limbs. Clin Exp Dermatol. 2007;32(1):132-134.
6. Kalsi H, Rahman A, Harbol T, Sidhu J. Giant hemosiderotic dermatofibroma: the largest giant dermatofibroma reported to date. Am J Dermatopathol. 2015;37(10):778-782.
7. Rosmaninho A, Farrajota P, Peixoto C, Amorim I, Selores M. Basal cell carcinoma overlying a dermatofibroma: a revisited controversy. Eur J Dermatol. 2011;21(1):137-138.
8. Sehgal VN, Sardana K, Khandpur S, Sharma S, Majumdar S, Aggarwal AK. Giant combined dermatofibroma with satellitosis. Clin Exp Dermatol. 2004;29(2):147-149.
9. Lang KJ, Lidder S, Hofer M, Graham C, Taylor A. Rapidly evolving giant dermatofibroma. Case Rep Med. 2010;2010:620910.
10. Micantonio T, Fargnoli MC, Peris K. Giant dermatofibroma appearing during pregnancy. Acta Derm Venereol. 2006;86(1):86-87.
11. Hueso L, Sanmartín O, Alfaro-Rubio A, et al. Giant dermatofibroma: case report and review of the literature. Actas Dermosifiliogr. 2007;98(2):121-124.
12. Chen TC, Kuo T, Chan HL. Dermatofibroma is a clonal proliferative disease. J Cutan Pathol. 2000;27(1):36-39.
13. Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. St. Louis, MO: W.B. Saunders; 2012:1962-1964.
14. Cribier B, Noacco G, Peltre B, Grosshans E. Stromelysin 3 expression: a useful marker for the differential diagnosis dermatofibroma versus dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2002;46(3):408-413.
15. Kamino H, Jacobson M. Dermatofibroma extending into the subcutaneous tissue. Differential diagnosis from dermatofibrosarcoma protuberans. Am J Surg Pathol. 1990;14(12):1156-1164.
 A 53-year-old otherwise healthy man was referred to us by his primary care provider for the evaluation of a large, asymptomatic, polypoid mass over his left lateral leg that he had for more than 25 years (Figure 1). He could not recall exactly how this lesion started, but said that it began as a very small bump similar to 2 other bumps on his legs. However, the lesion slowly grew, and about 15 years ago he traumatized it against a ledge, after which the lesion bled and “fell off.” It then began to grow again at a very slow rate and was completely asymptomatic. On examination, there was a 4.0 x 3.5 cm nontender, pink, firm, smooth surfaced, pedunculate, and exophytic mass over the left lateral leg. The lesion was clinically well circumscribed with a slightly indurated base.
A 53-year-old otherwise healthy man was referred to us by his primary care provider for the evaluation of a large, asymptomatic, polypoid mass over his left lateral leg that he had for more than 25 years (Figure 1). He could not recall exactly how this lesion started, but said that it began as a very small bump similar to 2 other bumps on his legs. However, the lesion slowly grew, and about 15 years ago he traumatized it against a ledge, after which the lesion bled and “fell off.” It then began to grow again at a very slow rate and was completely asymptomatic. On examination, there was a 4.0 x 3.5 cm nontender, pink, firm, smooth surfaced, pedunculate, and exophytic mass over the left lateral leg. The lesion was clinically well circumscribed with a slightly indurated base.
What is your diagnosis?
,
 A 53-year-old otherwise healthy man was referred to us by his primary care provider for the evaluation of a large, asymptomatic, polypoid mass over his left lateral leg that he had for more than 25 years (Figure 1). He could not recall exactly how this lesion started, but said that it began as a very small bump similar to 2 other bumps on his legs. However, the lesion slowly grew, and about 15 years ago he traumatized it against a ledge, after which the lesion bled and “fell off.” It then began to grow again at a very slow rate and was completely asymptomatic. On examination, there was a 4.0 x 3.5 cm nontender, pink, firm, smooth surfaced, pedunculate, and exophytic mass over the left lateral leg. The lesion was clinically well circumscribed with a slightly indurated base.
A 53-year-old otherwise healthy man was referred to us by his primary care provider for the evaluation of a large, asymptomatic, polypoid mass over his left lateral leg that he had for more than 25 years (Figure 1). He could not recall exactly how this lesion started, but said that it began as a very small bump similar to 2 other bumps on his legs. However, the lesion slowly grew, and about 15 years ago he traumatized it against a ledge, after which the lesion bled and “fell off.” It then began to grow again at a very slow rate and was completely asymptomatic. On examination, there was a 4.0 x 3.5 cm nontender, pink, firm, smooth surfaced, pedunculate, and exophytic mass over the left lateral leg. The lesion was clinically well circumscribed with a slightly indurated base.
What is your diagnosis?
To learn the answer, go to page 2
{{pagebreak}}
Diagnosis: Giant Dermatofibroma
Dermatofibroma (DF) is a common benign cutaneous lesion that occurs most frequently on the lower legs of women. Several clinical and pathological subtypes of DF have been described. Giant DF is a rare and unusual clinical variant that often presents as an exophytic or pedunculated mass.1 The large size and sometimes fungating appearance of these lesions may raise suspicion for malignancy. However, these lesions are histologically and biologically benign.
Several clinical and histologic variants of DF have been described, with many lesions containing combined features. Clinical variants include common DF, multiple DFs, eruptive DFs, atrophic DF, atypical polypoid DF, and giant DF.2 Multiple and/or eruptive DFs, either clustered or widespread, can be seen in autoimmune disorders (systemic lupus erythematosus, myasthenia gravis), in the setting of immunosuppression (HIV, infection), in atopic dermatitis, in pregnancy, as well as in a familial pattern.2,3 The other clinical subtypes are exceedingly rare, with limited case reports in the literature. Histologic variants are numerous and outlined in the Table. The histologic features of several variants may be found within the same lesion.4
Giant DF is a rare clinical variant of DF characterized by unusually large size, pedunculated appearance, benign behavior, and histopathologic features identical to that of common DFs. Although there is no clear definition of the minimal size required for the diagnosis of giant DF, several authors have used ≥3.5 cm to describe these lesions.2,5
The first case was reported in 1975 by Danckeart and Karassic as a 3.5 x 3.5 cm lesion on the pretibial area of a 52-year-old man. To date, 27 giant DFs have been reported, ranging from 3.5 to 30 cm.1,6,7
Clinical Presentations
DF usually presents as a slow-growing, small (≤1 cm), solitary, firm, and well-circumscribed dermal nodule with a brown or reddish-brown hue (Figure 1). The lesion often induces slight inward retraction of the overlying skin, and lateral compression may further exhibit this feature, creating a “dimple sign,” which indicates tethering of the overlying epidermis to the lesion. DFs are typically found on the legs but may also occur on various other parts of the body, and are more common in middle-aged women. They are usually asymptomatic, but can sometimes be pruritic or tender.
Although common DFs are easily recognized using only visual inspection and palpation, dermoscopy can help confirm the diagnosis. The classic finding under dermoscopy is a central white scar-like patch surrounded by a peripheral rim of hyperpigmentation.
Giant DF presents as a firm, exophytic, and often polypoid mass that may ulcerate or bleed. The surface may be smooth or keratotic. Purulent drainage, plaque-like presentation, and satellite lesions have also been reported features.8 Almost all of the cases have occurred on the legs, but they have also been reported in the groin,9 on the lower back of a pregnant woman,10 and on the posterior shoulder.11 Growth may be rapid or slow.9,10
Pathogenesis
The etiology of DFs is controversial. Many authors believe that they occur as a reactive process following some type of insult such as arthropod bite, trauma, folliculitis, or an inflammatory condition. Others consider DFs to be neoplastic processes due to the persistent nature of these lesions, as well as clonality of the proliferating cells.12 However, clonality has also been observed in various inflammatory conditions such as atopic dermatitis, psoriasis, and lichen sclerosis, thus this feature alone does not necessarily represent a neoplastic process.4
Whether DFs are reactive or neoplastic is still widely debated, however, most consider DFs to be reactive processes. The pathogenesis of giant DFs is even more obscure and only speculative.
 Histopathology
Histopathology
The histopathologic findings of giant DFs are fairly identical to those of common DFs. Typically, there is a fairly well-circumscribed dermal proliferation with aggregates of spindle-shaped fibroblasts arranged between thickened collagen bundles. The fibroblasts have plump nuclei, and although mitoses may be present, they are usually not numerous. Foamy histiocytes may be seen in variable numbers, and sometimes form multinucleated giant cells. There may be peripheral collagen trapping and an increased number of small blood vessels. DFs can become hemorrhagic with subsequent formation of hemosiderin deposits. The overlying epidermis is hyperplastic with hyperpigmentation of the basal layer. Sometimes follicular induction may be observed, where basaloid-appearing extensions emanate from the overlying epidermis and may mimic basal cell carcinoma (BCC).13 However, there have been some reports in the literature of BCC overlying a DF. Rosmaninho and colleagues7 reported a case where well-developed basaloid nests were seen extending from the epidermis and overlying a DF. They observed retraction artifact around the basaloid structures, positive Ber-EP4 staining, and even focal infiltration into the reticular dermis. This led to the diagnosis of BCC overlying a DF, and excision was performed. The differentiation between true BCC vs follicular induction overlying DFs remains a controversial topic.
Probably due to their large size, giant DFs may also have additional histologic features such as ulceration, areas of cystic degeneration, focal hemorrhage, and cholesterol clefting.4,9 It is especially important to distinguish giant DF from dermatofibrosarcoma protuberans (DFSP) histologically, and immunohistochemical markers are often helpful. DF is usually strongly positive for factor XIIIa, and negative for CD34. In contrast, factor XIIIa tends to be negative in DFSP, and CD34 tends to be positive. In one case, histopathologic examination demonstrated most conventional features of DF; however, there were some architectural features of DFSP. Staining with factor XIIIa and CD34 solidified the diagnosis of giant DF in that case.5 Despite the utility of these markers, there may still be variations in staining among different cases. Some DFs demonstrate focal CD34 reactivity, whereas some DFSPs paradoxically express factor XIIIa but not CD34.14 In such cases, stromelysin 3 (ST3) staining may be useful. Cribier and colleagues14 reported that of 40 cases of DFSP, none expressed ST3, while all 40 cases of DF including 10 giant DFs expressed ST3. In terms of architecture, Kamino and Jacobson15 described how to differentiate DF extending into the fat from DFSP, and found that DF primarily extends in an irregular fashion with a vertical or radial pattern, predominantly along the septa. They concluded that DF and DFSP had different patterns of fat extension. Thus, the architecture of the lesion along with characteristic immunostaining patterns help to differentiate DF from DFSP.
Differential Diagnosis
Given the large tumor-like appearance of giant DF, it can often be mistaken for malignant lesions. The most important entity in the differential diagnosis is DFSP. Other lesions that may present similarly include sarcoma, BCC, squamous cell carcinoma, melanoma, eccrine acrospiroma, fibroma, pyogenic granuloma, keloid, malignant fibrous histiocytoma, and collagenoma.
Treatment and Prognosis
Giant DF has a benign course, and surgical excision is curative. However, removal of the lesion is not necessary unless there is diagnostic uncertainty or patient discomfort.4 To date, recurrence after surgical excision has not been reported, even after incomplete excision.2,4,6
Our Patient
A shave removal of the lesion was performed with a deep-shave technique. The base was then electrodesiccated. Microscopic examination of the lesion revealed a bland spindle cell proliferation consistent with DF (Figures 2A and B). At 2-week follow-up, the wound was healing well. The patient was informed of the benign etiology of the mass, and that complete excision was an option but not a requirement. With the understanding that there is a possibility of local recurrence, the patient deferred excision at this time. He will be monitored periodically for any evidence of recurrence.
Conclusion
 Giant DF is a rare clinical variant of DF characterized by its unusually large size, with fewer than 30 cases reported to date. It can often be misdiagnosed as a malignancy due to its striking appearance. However, clinicians should be aware that giant DF is a benign process and that surgical excision, though not required, is curative. To our knowledge, recurrence after excision of a giant DF has not been reported, nor have there been reports of diagnosis revision to DFSP after re-excision. However, the possibility of missing the diagnosis of DFSP with a superficial biopsy still remains a valid concern for dermatologists and dermatologic surgeons.
Giant DF is a rare clinical variant of DF characterized by its unusually large size, with fewer than 30 cases reported to date. It can often be misdiagnosed as a malignancy due to its striking appearance. However, clinicians should be aware that giant DF is a benign process and that surgical excision, though not required, is curative. To our knowledge, recurrence after excision of a giant DF has not been reported, nor have there been reports of diagnosis revision to DFSP after re-excision. However, the possibility of missing the diagnosis of DFSP with a superficial biopsy still remains a valid concern for dermatologists and dermatologic surgeons.
 Dr Liu is with the department of dermatology at the State University of New York Downstate Medical Center in Brooklyn, NY.
Dr Liu is with the department of dermatology at the State University of New York Downstate Medical Center in Brooklyn, NY.
Dr Alapati is with the department of dermatology at Veteran Affairs Medical Center in Brooklyn, NY.
Dr Khachemoune, the Section Editor of Derm DX, is with the department of dermatology at Veteran Affairs Medical Center, and the department of dermatology at the State University of New York Downstate, both in Brooklyn, NY.
Disclosure: The authors report no relevant financial relationships.
References
1. Danckaert KB, Karassik SL. Dermatofibroma: an unusual presentation. Cutis. 1975;16:245-247.
2. Requena L, Fariña MC, Fuente C, et al. Giant dermatofibroma. A little-known clinical variant of dermatofibroma. J Am Acad Dermatol. 1994;30 (5 Pt 1):714-718.
3. Niiyama S, Katsuoka K, Happle R, Hoffmann R. Multiple eruptive dermatofibromas: a review of the literature. Acta Derm Venereol. 2002;82(4):241-244.
4. Pusztaszeri M, Jaquet PY, Williamson C. Giant hemosiderotic dermatofibroma: a case report and review of the literature. Case Rep Dermatol. 2011;18;3(1):32-36.
5. Hoshina D, Shibaki A, Aoyagi S, Kimura K, Shimizu H. Giant dermatofibroma: a rare variant of dermatofibroma preferentially developing on the lower limbs. Clin Exp Dermatol. 2007;32(1):132-134.
6. Kalsi H, Rahman A, Harbol T, Sidhu J. Giant hemosiderotic dermatofibroma: the largest giant dermatofibroma reported to date. Am J Dermatopathol. 2015;37(10):778-782.
7. Rosmaninho A, Farrajota P, Peixoto C, Amorim I, Selores M. Basal cell carcinoma overlying a dermatofibroma: a revisited controversy. Eur J Dermatol. 2011;21(1):137-138.
8. Sehgal VN, Sardana K, Khandpur S, Sharma S, Majumdar S, Aggarwal AK. Giant combined dermatofibroma with satellitosis. Clin Exp Dermatol. 2004;29(2):147-149.
9. Lang KJ, Lidder S, Hofer M, Graham C, Taylor A. Rapidly evolving giant dermatofibroma. Case Rep Med. 2010;2010:620910.
10. Micantonio T, Fargnoli MC, Peris K. Giant dermatofibroma appearing during pregnancy. Acta Derm Venereol. 2006;86(1):86-87.
11. Hueso L, Sanmartín O, Alfaro-Rubio A, et al. Giant dermatofibroma: case report and review of the literature. Actas Dermosifiliogr. 2007;98(2):121-124.
12. Chen TC, Kuo T, Chan HL. Dermatofibroma is a clonal proliferative disease. J Cutan Pathol. 2000;27(1):36-39.
13. Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. St. Louis, MO: W.B. Saunders; 2012:1962-1964.
14. Cribier B, Noacco G, Peltre B, Grosshans E. Stromelysin 3 expression: a useful marker for the differential diagnosis dermatofibroma versus dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2002;46(3):408-413.
15. Kamino H, Jacobson M. Dermatofibroma extending into the subcutaneous tissue. Differential diagnosis from dermatofibrosarcoma protuberans. Am J Surg Pathol. 1990;14(12):1156-1164.
 A 53-year-old otherwise healthy man was referred to us by his primary care provider for the evaluation of a large, asymptomatic, polypoid mass over his left lateral leg that he had for more than 25 years (Figure 1). He could not recall exactly how this lesion started, but said that it began as a very small bump similar to 2 other bumps on his legs. However, the lesion slowly grew, and about 15 years ago he traumatized it against a ledge, after which the lesion bled and “fell off.” It then began to grow again at a very slow rate and was completely asymptomatic. On examination, there was a 4.0 x 3.5 cm nontender, pink, firm, smooth surfaced, pedunculate, and exophytic mass over the left lateral leg. The lesion was clinically well circumscribed with a slightly indurated base.
A 53-year-old otherwise healthy man was referred to us by his primary care provider for the evaluation of a large, asymptomatic, polypoid mass over his left lateral leg that he had for more than 25 years (Figure 1). He could not recall exactly how this lesion started, but said that it began as a very small bump similar to 2 other bumps on his legs. However, the lesion slowly grew, and about 15 years ago he traumatized it against a ledge, after which the lesion bled and “fell off.” It then began to grow again at a very slow rate and was completely asymptomatic. On examination, there was a 4.0 x 3.5 cm nontender, pink, firm, smooth surfaced, pedunculate, and exophytic mass over the left lateral leg. The lesion was clinically well circumscribed with a slightly indurated base.
What is your diagnosis?
Diagnosis: Giant Dermatofibroma
Dermatofibroma (DF) is a common benign cutaneous lesion that occurs most frequently on the lower legs of women. Several clinical and pathological subtypes of DF have been described. Giant DF is a rare and unusual clinical variant that often presents as an exophytic or pedunculated mass.1 The large size and sometimes fungating appearance of these lesions may raise suspicion for malignancy. However, these lesions are histologically and biologically benign.
Several clinical and histologic variants of DF have been described, with many lesions containing combined features. Clinical variants include common DF, multiple DFs, eruptive DFs, atrophic DF, atypical polypoid DF, and giant DF.2 Multiple and/or eruptive DFs, either clustered or widespread, can be seen in autoimmune disorders (systemic lupus erythematosus, myasthenia gravis), in the setting of immunosuppression (HIV, infection), in atopic dermatitis, in pregnancy, as well as in a familial pattern.2,3 The other clinical subtypes are exceedingly rare, with limited case reports in the literature. Histologic variants are numerous and outlined in the Table. The histologic features of several variants may be found within the same lesion.4
Giant DF is a rare clinical variant of DF characterized by unusually large size, pedunculated appearance, benign behavior, and histopathologic features identical to that of common DFs. Although there is no clear definition of the minimal size required for the diagnosis of giant DF, several authors have used ≥3.5 cm to describe these lesions.2,5
The first case was reported in 1975 by Danckeart and Karassic as a 3.5 x 3.5 cm lesion on the pretibial area of a 52-year-old man. To date, 27 giant DFs have been reported, ranging from 3.5 to 30 cm.1,6,7
Clinical Presentations
DF usually presents as a slow-growing, small (≤1 cm), solitary, firm, and well-circumscribed dermal nodule with a brown or reddish-brown hue (Figure 1). The lesion often induces slight inward retraction of the overlying skin, and lateral compression may further exhibit this feature, creating a “dimple sign,” which indicates tethering of the overlying epidermis to the lesion. DFs are typically found on the legs but may also occur on various other parts of the body, and are more common in middle-aged women. They are usually asymptomatic, but can sometimes be pruritic or tender.
Although common DFs are easily recognized using only visual inspection and palpation, dermoscopy can help confirm the diagnosis. The classic finding under dermoscopy is a central white scar-like patch surrounded by a peripheral rim of hyperpigmentation.
Giant DF presents as a firm, exophytic, and often polypoid mass that may ulcerate or bleed. The surface may be smooth or keratotic. Purulent drainage, plaque-like presentation, and satellite lesions have also been reported features.8 Almost all of the cases have occurred on the legs, but they have also been reported in the groin,9 on the lower back of a pregnant woman,10 and on the posterior shoulder.11 Growth may be rapid or slow.9,10
Pathogenesis
The etiology of DFs is controversial. Many authors believe that they occur as a reactive process following some type of insult such as arthropod bite, trauma, folliculitis, or an inflammatory condition. Others consider DFs to be neoplastic processes due to the persistent nature of these lesions, as well as clonality of the proliferating cells.12 However, clonality has also been observed in various inflammatory conditions such as atopic dermatitis, psoriasis, and lichen sclerosis, thus this feature alone does not necessarily represent a neoplastic process.4
Whether DFs are reactive or neoplastic is still widely debated, however, most consider DFs to be reactive processes. The pathogenesis of giant DFs is even more obscure and only speculative.
 Histopathology
Histopathology
The histopathologic findings of giant DFs are fairly identical to those of common DFs. Typically, there is a fairly well-circumscribed dermal proliferation with aggregates of spindle-shaped fibroblasts arranged between thickened collagen bundles. The fibroblasts have plump nuclei, and although mitoses may be present, they are usually not numerous. Foamy histiocytes may be seen in variable numbers, and sometimes form multinucleated giant cells. There may be peripheral collagen trapping and an increased number of small blood vessels. DFs can become hemorrhagic with subsequent formation of hemosiderin deposits. The overlying epidermis is hyperplastic with hyperpigmentation of the basal layer. Sometimes follicular induction may be observed, where basaloid-appearing extensions emanate from the overlying epidermis and may mimic basal cell carcinoma (BCC).13 However, there have been some reports in the literature of BCC overlying a DF. Rosmaninho and colleagues7 reported a case where well-developed basaloid nests were seen extending from the epidermis and overlying a DF. They observed retraction artifact around the basaloid structures, positive Ber-EP4 staining, and even focal infiltration into the reticular dermis. This led to the diagnosis of BCC overlying a DF, and excision was performed. The differentiation between true BCC vs follicular induction overlying DFs remains a controversial topic.
Probably due to their large size, giant DFs may also have additional histologic features such as ulceration, areas of cystic degeneration, focal hemorrhage, and cholesterol clefting.4,9 It is especially important to distinguish giant DF from dermatofibrosarcoma protuberans (DFSP) histologically, and immunohistochemical markers are often helpful. DF is usually strongly positive for factor XIIIa, and negative for CD34. In contrast, factor XIIIa tends to be negative in DFSP, and CD34 tends to be positive. In one case, histopathologic examination demonstrated most conventional features of DF; however, there were some architectural features of DFSP. Staining with factor XIIIa and CD34 solidified the diagnosis of giant DF in that case.5 Despite the utility of these markers, there may still be variations in staining among different cases. Some DFs demonstrate focal CD34 reactivity, whereas some DFSPs paradoxically express factor XIIIa but not CD34.14 In such cases, stromelysin 3 (ST3) staining may be useful. Cribier and colleagues14 reported that of 40 cases of DFSP, none expressed ST3, while all 40 cases of DF including 10 giant DFs expressed ST3. In terms of architecture, Kamino and Jacobson15 described how to differentiate DF extending into the fat from DFSP, and found that DF primarily extends in an irregular fashion with a vertical or radial pattern, predominantly along the septa. They concluded that DF and DFSP had different patterns of fat extension. Thus, the architecture of the lesion along with characteristic immunostaining patterns help to differentiate DF from DFSP.
Differential Diagnosis
Given the large tumor-like appearance of giant DF, it can often be mistaken for malignant lesions. The most important entity in the differential diagnosis is DFSP. Other lesions that may present similarly include sarcoma, BCC, squamous cell carcinoma, melanoma, eccrine acrospiroma, fibroma, pyogenic granuloma, keloid, malignant fibrous histiocytoma, and collagenoma.
Treatment and Prognosis
Giant DF has a benign course, and surgical excision is curative. However, removal of the lesion is not necessary unless there is diagnostic uncertainty or patient discomfort.4 To date, recurrence after surgical excision has not been reported, even after incomplete excision.2,4,6
Our Patient
A shave removal of the lesion was performed with a deep-shave technique. The base was then electrodesiccated. Microscopic examination of the lesion revealed a bland spindle cell proliferation consistent with DF (Figures 2A and B). At 2-week follow-up, the wound was healing well. The patient was informed of the benign etiology of the mass, and that complete excision was an option but not a requirement. With the understanding that there is a possibility of local recurrence, the patient deferred excision at this time. He will be monitored periodically for any evidence of recurrence.
Conclusion
 Giant DF is a rare clinical variant of DF characterized by its unusually large size, with fewer than 30 cases reported to date. It can often be misdiagnosed as a malignancy due to its striking appearance. However, clinicians should be aware that giant DF is a benign process and that surgical excision, though not required, is curative. To our knowledge, recurrence after excision of a giant DF has not been reported, nor have there been reports of diagnosis revision to DFSP after re-excision. However, the possibility of missing the diagnosis of DFSP with a superficial biopsy still remains a valid concern for dermatologists and dermatologic surgeons.
Giant DF is a rare clinical variant of DF characterized by its unusually large size, with fewer than 30 cases reported to date. It can often be misdiagnosed as a malignancy due to its striking appearance. However, clinicians should be aware that giant DF is a benign process and that surgical excision, though not required, is curative. To our knowledge, recurrence after excision of a giant DF has not been reported, nor have there been reports of diagnosis revision to DFSP after re-excision. However, the possibility of missing the diagnosis of DFSP with a superficial biopsy still remains a valid concern for dermatologists and dermatologic surgeons.
 Dr Liu is with the department of dermatology at the State University of New York Downstate Medical Center in Brooklyn, NY.
Dr Liu is with the department of dermatology at the State University of New York Downstate Medical Center in Brooklyn, NY.
Dr Alapati is with the department of dermatology at Veteran Affairs Medical Center in Brooklyn, NY.
Dr Khachemoune, the Section Editor of Derm DX, is with the department of dermatology at Veteran Affairs Medical Center, and the department of dermatology at the State University of New York Downstate, both in Brooklyn, NY.
Disclosure: The authors report no relevant financial relationships.
References
1. Danckaert KB, Karassik SL. Dermatofibroma: an unusual presentation. Cutis. 1975;16:245-247.
2. Requena L, Fariña MC, Fuente C, et al. Giant dermatofibroma. A little-known clinical variant of dermatofibroma. J Am Acad Dermatol. 1994;30 (5 Pt 1):714-718.
3. Niiyama S, Katsuoka K, Happle R, Hoffmann R. Multiple eruptive dermatofibromas: a review of the literature. Acta Derm Venereol. 2002;82(4):241-244.
4. Pusztaszeri M, Jaquet PY, Williamson C. Giant hemosiderotic dermatofibroma: a case report and review of the literature. Case Rep Dermatol. 2011;18;3(1):32-36.
5. Hoshina D, Shibaki A, Aoyagi S, Kimura K, Shimizu H. Giant dermatofibroma: a rare variant of dermatofibroma preferentially developing on the lower limbs. Clin Exp Dermatol. 2007;32(1):132-134.
6. Kalsi H, Rahman A, Harbol T, Sidhu J. Giant hemosiderotic dermatofibroma: the largest giant dermatofibroma reported to date. Am J Dermatopathol. 2015;37(10):778-782.
7. Rosmaninho A, Farrajota P, Peixoto C, Amorim I, Selores M. Basal cell carcinoma overlying a dermatofibroma: a revisited controversy. Eur J Dermatol. 2011;21(1):137-138.
8. Sehgal VN, Sardana K, Khandpur S, Sharma S, Majumdar S, Aggarwal AK. Giant combined dermatofibroma with satellitosis. Clin Exp Dermatol. 2004;29(2):147-149.
9. Lang KJ, Lidder S, Hofer M, Graham C, Taylor A. Rapidly evolving giant dermatofibroma. Case Rep Med. 2010;2010:620910.
10. Micantonio T, Fargnoli MC, Peris K. Giant dermatofibroma appearing during pregnancy. Acta Derm Venereol. 2006;86(1):86-87.
11. Hueso L, Sanmartín O, Alfaro-Rubio A, et al. Giant dermatofibroma: case report and review of the literature. Actas Dermosifiliogr. 2007;98(2):121-124.
12. Chen TC, Kuo T, Chan HL. Dermatofibroma is a clonal proliferative disease. J Cutan Pathol. 2000;27(1):36-39.
13. Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. St. Louis, MO: W.B. Saunders; 2012:1962-1964.
14. Cribier B, Noacco G, Peltre B, Grosshans E. Stromelysin 3 expression: a useful marker for the differential diagnosis dermatofibroma versus dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2002;46(3):408-413.
15. Kamino H, Jacobson M. Dermatofibroma extending into the subcutaneous tissue. Differential diagnosis from dermatofibrosarcoma protuberans. Am J Surg Pathol. 1990;14(12):1156-1164.
Diagnosis: Giant Dermatofibroma
Dermatofibroma (DF) is a common benign cutaneous lesion that occurs most frequently on the lower legs of women. Several clinical and pathological subtypes of DF have been described. Giant DF is a rare and unusual clinical variant that often presents as an exophytic or pedunculated mass.1 The large size and sometimes fungating appearance of these lesions may raise suspicion for malignancy. However, these lesions are histologically and biologically benign.
Several clinical and histologic variants of DF have been described, with many lesions containing combined features. Clinical variants include common DF, multiple DFs, eruptive DFs, atrophic DF, atypical polypoid DF, and giant DF.2 Multiple and/or eruptive DFs, either clustered or widespread, can be seen in autoimmune disorders (systemic lupus erythematosus, myasthenia gravis), in the setting of immunosuppression (HIV, infection), in atopic dermatitis, in pregnancy, as well as in a familial pattern.2,3 The other clinical subtypes are exceedingly rare, with limited case reports in the literature. Histologic variants are numerous and outlined in the Table. The histologic features of several variants may be found within the same lesion.4
Giant DF is a rare clinical variant of DF characterized by unusually large size, pedunculated appearance, benign behavior, and histopathologic features identical to that of common DFs. Although there is no clear definition of the minimal size required for the diagnosis of giant DF, several authors have used ≥3.5 cm to describe these lesions.2,5
The first case was reported in 1975 by Danckeart and Karassic as a 3.5 x 3.5 cm lesion on the pretibial area of a 52-year-old man. To date, 27 giant DFs have been reported, ranging from 3.5 to 30 cm.1,6,7
Clinical Presentations
DF usually presents as a slow-growing, small (≤1 cm), solitary, firm, and well-circumscribed dermal nodule with a brown or reddish-brown hue (Figure 1). The lesion often induces slight inward retraction of the overlying skin, and lateral compression may further exhibit this feature, creating a “dimple sign,” which indicates tethering of the overlying epidermis to the lesion. DFs are typically found on the legs but may also occur on various other parts of the body, and are more common in middle-aged women. They are usually asymptomatic, but can sometimes be pruritic or tender.
Although common DFs are easily recognized using only visual inspection and palpation, dermoscopy can help confirm the diagnosis. The classic finding under dermoscopy is a central white scar-like patch surrounded by a peripheral rim of hyperpigmentation.
Giant DF presents as a firm, exophytic, and often polypoid mass that may ulcerate or bleed. The surface may be smooth or keratotic. Purulent drainage, plaque-like presentation, and satellite lesions have also been reported features.8 Almost all of the cases have occurred on the legs, but they have also been reported in the groin,9 on the lower back of a pregnant woman,10 and on the posterior shoulder.11 Growth may be rapid or slow.9,10
Pathogenesis
The etiology of DFs is controversial. Many authors believe that they occur as a reactive process following some type of insult such as arthropod bite, trauma, folliculitis, or an inflammatory condition. Others consider DFs to be neoplastic processes due to the persistent nature of these lesions, as well as clonality of the proliferating cells.12 However, clonality has also been observed in various inflammatory conditions such as atopic dermatitis, psoriasis, and lichen sclerosis, thus this feature alone does not necessarily represent a neoplastic process.4
Whether DFs are reactive or neoplastic is still widely debated, however, most consider DFs to be reactive processes. The pathogenesis of giant DFs is even more obscure and only speculative.
 Histopathology
Histopathology
The histopathologic findings of giant DFs are fairly identical to those of common DFs. Typically, there is a fairly well-circumscribed dermal proliferation with aggregates of spindle-shaped fibroblasts arranged between thickened collagen bundles. The fibroblasts have plump nuclei, and although mitoses may be present, they are usually not numerous. Foamy histiocytes may be seen in variable numbers, and sometimes form multinucleated giant cells. There may be peripheral collagen trapping and an increased number of small blood vessels. DFs can become hemorrhagic with subsequent formation of hemosiderin deposits. The overlying epidermis is hyperplastic with hyperpigmentation of the basal layer. Sometimes follicular induction may be observed, where basaloid-appearing extensions emanate from the overlying epidermis and may mimic basal cell carcinoma (BCC).13 However, there have been some reports in the literature of BCC overlying a DF. Rosmaninho and colleagues7 reported a case where well-developed basaloid nests were seen extending from the epidermis and overlying a DF. They observed retraction artifact around the basaloid structures, positive Ber-EP4 staining, and even focal infiltration into the reticular dermis. This led to the diagnosis of BCC overlying a DF, and excision was performed. The differentiation between true BCC vs follicular induction overlying DFs remains a controversial topic.
Probably due to their large size, giant DFs may also have additional histologic features such as ulceration, areas of cystic degeneration, focal hemorrhage, and cholesterol clefting.4,9 It is especially important to distinguish giant DF from dermatofibrosarcoma protuberans (DFSP) histologically, and immunohistochemical markers are often helpful. DF is usually strongly positive for factor XIIIa, and negative for CD34. In contrast, factor XIIIa tends to be negative in DFSP, and CD34 tends to be positive. In one case, histopathologic examination demonstrated most conventional features of DF; however, there were some architectural features of DFSP. Staining with factor XIIIa and CD34 solidified the diagnosis of giant DF in that case.5 Despite the utility of these markers, there may still be variations in staining among different cases. Some DFs demonstrate focal CD34 reactivity, whereas some DFSPs paradoxically express factor XIIIa but not CD34.14 In such cases, stromelysin 3 (ST3) staining may be useful. Cribier and colleagues14 reported that of 40 cases of DFSP, none expressed ST3, while all 40 cases of DF including 10 giant DFs expressed ST3. In terms of architecture, Kamino and Jacobson15 described how to differentiate DF extending into the fat from DFSP, and found that DF primarily extends in an irregular fashion with a vertical or radial pattern, predominantly along the septa. They concluded that DF and DFSP had different patterns of fat extension. Thus, the architecture of the lesion along with characteristic immunostaining patterns help to differentiate DF from DFSP.
Differential Diagnosis
Given the large tumor-like appearance of giant DF, it can often be mistaken for malignant lesions. The most important entity in the differential diagnosis is DFSP. Other lesions that may present similarly include sarcoma, BCC, squamous cell carcinoma, melanoma, eccrine acrospiroma, fibroma, pyogenic granuloma, keloid, malignant fibrous histiocytoma, and collagenoma.
Treatment and Prognosis
Giant DF has a benign course, and surgical excision is curative. However, removal of the lesion is not necessary unless there is diagnostic uncertainty or patient discomfort.4 To date, recurrence after surgical excision has not been reported, even after incomplete excision.2,4,6
Our Patient
A shave removal of the lesion was performed with a deep-shave technique. The base was then electrodesiccated. Microscopic examination of the lesion revealed a bland spindle cell proliferation consistent with DF (Figures 2A and B). At 2-week follow-up, the wound was healing well. The patient was informed of the benign etiology of the mass, and that complete excision was an option but not a requirement. With the understanding that there is a possibility of local recurrence, the patient deferred excision at this time. He will be monitored periodically for any evidence of recurrence.
Conclusion
 Giant DF is a rare clinical variant of DF characterized by its unusually large size, with fewer than 30 cases reported to date. It can often be misdiagnosed as a malignancy due to its striking appearance. However, clinicians should be aware that giant DF is a benign process and that surgical excision, though not required, is curative. To our knowledge, recurrence after excision of a giant DF has not been reported, nor have there been reports of diagnosis revision to DFSP after re-excision. However, the possibility of missing the diagnosis of DFSP with a superficial biopsy still remains a valid concern for dermatologists and dermatologic surgeons.
Giant DF is a rare clinical variant of DF characterized by its unusually large size, with fewer than 30 cases reported to date. It can often be misdiagnosed as a malignancy due to its striking appearance. However, clinicians should be aware that giant DF is a benign process and that surgical excision, though not required, is curative. To our knowledge, recurrence after excision of a giant DF has not been reported, nor have there been reports of diagnosis revision to DFSP after re-excision. However, the possibility of missing the diagnosis of DFSP with a superficial biopsy still remains a valid concern for dermatologists and dermatologic surgeons.
 Dr Liu is with the department of dermatology at the State University of New York Downstate Medical Center in Brooklyn, NY.
Dr Liu is with the department of dermatology at the State University of New York Downstate Medical Center in Brooklyn, NY.
Dr Alapati is with the department of dermatology at Veteran Affairs Medical Center in Brooklyn, NY.
Dr Khachemoune, the Section Editor of Derm DX, is with the department of dermatology at Veteran Affairs Medical Center, and the department of dermatology at the State University of New York Downstate, both in Brooklyn, NY.
Disclosure: The authors report no relevant financial relationships.
References
1. Danckaert KB, Karassik SL. Dermatofibroma: an unusual presentation. Cutis. 1975;16:245-247.
2. Requena L, Fariña MC, Fuente C, et al. Giant dermatofibroma. A little-known clinical variant of dermatofibroma. J Am Acad Dermatol. 1994;30 (5 Pt 1):714-718.
3. Niiyama S, Katsuoka K, Happle R, Hoffmann R. Multiple eruptive dermatofibromas: a review of the literature. Acta Derm Venereol. 2002;82(4):241-244.
4. Pusztaszeri M, Jaquet PY, Williamson C. Giant hemosiderotic dermatofibroma: a case report and review of the literature. Case Rep Dermatol. 2011;18;3(1):32-36.
5. Hoshina D, Shibaki A, Aoyagi S, Kimura K, Shimizu H. Giant dermatofibroma: a rare variant of dermatofibroma preferentially developing on the lower limbs. Clin Exp Dermatol. 2007;32(1):132-134.
6. Kalsi H, Rahman A, Harbol T, Sidhu J. Giant hemosiderotic dermatofibroma: the largest giant dermatofibroma reported to date. Am J Dermatopathol. 2015;37(10):778-782.
7. Rosmaninho A, Farrajota P, Peixoto C, Amorim I, Selores M. Basal cell carcinoma overlying a dermatofibroma: a revisited controversy. Eur J Dermatol. 2011;21(1):137-138.
8. Sehgal VN, Sardana K, Khandpur S, Sharma S, Majumdar S, Aggarwal AK. Giant combined dermatofibroma with satellitosis. Clin Exp Dermatol. 2004;29(2):147-149.
9. Lang KJ, Lidder S, Hofer M, Graham C, Taylor A. Rapidly evolving giant dermatofibroma. Case Rep Med. 2010;2010:620910.
10. Micantonio T, Fargnoli MC, Peris K. Giant dermatofibroma appearing during pregnancy. Acta Derm Venereol. 2006;86(1):86-87.
11. Hueso L, Sanmartín O, Alfaro-Rubio A, et al. Giant dermatofibroma: case report and review of the literature. Actas Dermosifiliogr. 2007;98(2):121-124.
12. Chen TC, Kuo T, Chan HL. Dermatofibroma is a clonal proliferative disease. J Cutan Pathol. 2000;27(1):36-39.
13. Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. St. Louis, MO: W.B. Saunders; 2012:1962-1964.
14. Cribier B, Noacco G, Peltre B, Grosshans E. Stromelysin 3 expression: a useful marker for the differential diagnosis dermatofibroma versus dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2002;46(3):408-413.
15. Kamino H, Jacobson M. Dermatofibroma extending into the subcutaneous tissue. Differential diagnosis from dermatofibrosarcoma protuberans. Am J Surg Pathol. 1990;14(12):1156-1164.
 A 53-year-old otherwise healthy man was referred to us by his primary care provider for the evaluation of a large, asymptomatic, polypoid mass over his left lateral leg that he had for more than 25 years (Figure 1). He could not recall exactly how this lesion started, but said that it began as a very small bump similar to 2 other bumps on his legs. However, the lesion slowly grew, and about 15 years ago he traumatized it against a ledge, after which the lesion bled and “fell off.” It then began to grow again at a very slow rate and was completely asymptomatic. On examination, there was a 4.0 x 3.5 cm nontender, pink, firm, smooth surfaced, pedunculate, and exophytic mass over the left lateral leg. The lesion was clinically well circumscribed with a slightly indurated base.
A 53-year-old otherwise healthy man was referred to us by his primary care provider for the evaluation of a large, asymptomatic, polypoid mass over his left lateral leg that he had for more than 25 years (Figure 1). He could not recall exactly how this lesion started, but said that it began as a very small bump similar to 2 other bumps on his legs. However, the lesion slowly grew, and about 15 years ago he traumatized it against a ledge, after which the lesion bled and “fell off.” It then began to grow again at a very slow rate and was completely asymptomatic. On examination, there was a 4.0 x 3.5 cm nontender, pink, firm, smooth surfaced, pedunculate, and exophytic mass over the left lateral leg. The lesion was clinically well circumscribed with a slightly indurated base.





 A 53-year-old otherwise healthy man was referred to us by his primary care provider for the evaluation of a large, asymptomatic, polypoid mass over his left lateral leg that he had for more than 25 years (Figure 1). He could not recall exactly how this lesion started, but said that it began as a very small bump similar to 2 other bumps on his legs. However, the lesion slowly grew, and about 15 years ago he traumatized it against a ledge, after which the lesion bled and “fell off.” It then began to grow again at a very slow rate and was completely asymptomatic. On examination, there was a 4.0 x 3.5 cm nontender, pink, firm, smooth surfaced, pedunculate, and exophytic mass over the left lateral leg. The lesion was clinically well circumscribed with a slightly indurated base.
A 53-year-old otherwise healthy man was referred to us by his primary care provider for the evaluation of a large, asymptomatic, polypoid mass over his left lateral leg that he had for more than 25 years (Figure 1). He could not recall exactly how this lesion started, but said that it began as a very small bump similar to 2 other bumps on his legs. However, the lesion slowly grew, and about 15 years ago he traumatized it against a ledge, after which the lesion bled and “fell off.” It then began to grow again at a very slow rate and was completely asymptomatic. On examination, there was a 4.0 x 3.5 cm nontender, pink, firm, smooth surfaced, pedunculate, and exophytic mass over the left lateral leg. The lesion was clinically well circumscribed with a slightly indurated base. Histopathology
Histopathology Giant DF is a rare clinical variant of DF characterized by its unusually large size, with fewer than 30 cases reported to date. It can often be misdiagnosed as a malignancy due to its striking appearance. However, clinicians should be aware that giant DF is a benign process and that surgical excision, though not required, is curative. To our knowledge, recurrence after excision of a giant DF has not been reported, nor have there been reports of diagnosis revision to DFSP after re-excision. However, the possibility of missing the diagnosis of DFSP with a superficial biopsy still remains a valid concern for dermatologists and dermatologic surgeons.
Giant DF is a rare clinical variant of DF characterized by its unusually large size, with fewer than 30 cases reported to date. It can often be misdiagnosed as a malignancy due to its striking appearance. However, clinicians should be aware that giant DF is a benign process and that surgical excision, though not required, is curative. To our knowledge, recurrence after excision of a giant DF has not been reported, nor have there been reports of diagnosis revision to DFSP after re-excision. However, the possibility of missing the diagnosis of DFSP with a superficial biopsy still remains a valid concern for dermatologists and dermatologic surgeons. Dr Liu is with the department of dermatology at the State University of New York Downstate Medical Center in Brooklyn, NY.
Dr Liu is with the department of dermatology at the State University of New York Downstate Medical Center in Brooklyn, NY. Histopathology
Histopathology Giant DF is a rare clinical variant of DF characterized by its unusually large size, with fewer than 30 cases reported to date. It can often be misdiagnosed as a malignancy due to its striking appearance. However, clinicians should be aware that giant DF is a benign process and that surgical excision, though not required, is curative. To our knowledge, recurrence after excision of a giant DF has not been reported, nor have there been reports of diagnosis revision to DFSP after re-excision. However, the possibility of missing the diagnosis of DFSP with a superficial biopsy still remains a valid concern for dermatologists and dermatologic surgeons.
Giant DF is a rare clinical variant of DF characterized by its unusually large size, with fewer than 30 cases reported to date. It can often be misdiagnosed as a malignancy due to its striking appearance. However, clinicians should be aware that giant DF is a benign process and that surgical excision, though not required, is curative. To our knowledge, recurrence after excision of a giant DF has not been reported, nor have there been reports of diagnosis revision to DFSP after re-excision. However, the possibility of missing the diagnosis of DFSP with a superficial biopsy still remains a valid concern for dermatologists and dermatologic surgeons. Dr Liu is with the department of dermatology at the State University of New York Downstate Medical Center in Brooklyn, NY.
Dr Liu is with the department of dermatology at the State University of New York Downstate Medical Center in Brooklyn, NY.

















