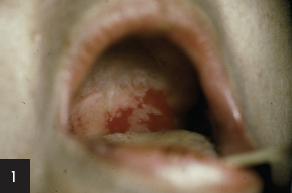
 1. This patient’s serum contains autoantibodies to desmoglein 3 and desmoglein 1. The diagnosis is:
1. This patient’s serum contains autoantibodies to desmoglein 3 and desmoglein 1. The diagnosis is:
a) Pemphigus vulgaris localized to mucous membranes
b) Generalized pemphigus vulgaris
c) Generalized pemphigus foliaceus
d) Mixed pemphigus vulgaris and pemphigus foliaceus
e) Paraneoplastic pemphigus
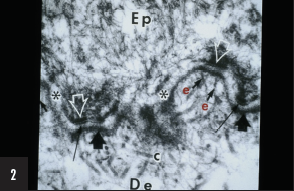 2. The structure labeled “e” is responsible for:
2. The structure labeled “e” is responsible for:
a) Keratinocyte-keratinocyte adhesion
b) Keratinocyte-lamina densa adhesion
c) Lamina densa-dermal adhesion
d) Keratinocyte-dermal adhesion
e) Lamina densa-elastic fiber adhesion
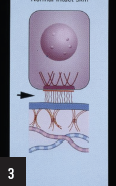 3. Which of the following proteins is located within the region of the dermoepidermal junction indicated by the arrow?
3. Which of the following proteins is located within the region of the dermoepidermal junction indicated by the arrow?
a) Laminin-332 (formerly named laminin-5)
b) Plectin
c) Keratin 5
d) Type IV collagen
e) Type VII collagen
To learn the answers, go to page 2
{{pagebreak}}
Board Review Answers:
1. This patient’s serum contains autoantibodies to desmoglein 3 and desmoglein i.
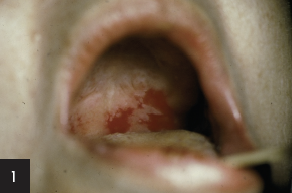 The diagnosis is:
The diagnosis is:
b) Generalized pemphigus vulgaris
Recent studies have provided an immunologic explanation (“desmoglein compensation theory”) for why some patients with pemphigus vulgaris have only mucosal lesions, while others have both mucosal and cutaneous lesions and why mucosal lesions do not occur in pemphigus foliaceus.
Pemphigus vulgaris patients with mucosal lesions, without significant skin lesions, have autoantibodies only to desmoglein 3. Pemphigus foliaceus patients lack mucosal involvement because they have autoantibodies only to desmoglein 1 and there is very little of that protein in the oral cavity. Coexistent desmoglein 3 stabilizes that tissue in pemphigus foliaceus. Desmoglein 3 is found on both mucosal and nonmucosal epithelial cells, but also appears to be the major cadhedrin keratinocyte adhesion protein on mucosal epithelial surfaces. Pemphigus vulgaris patients having both mucosal and skin lesions have autoantibodies to both desmoglein 3 and desmoglein 1. Desmoglein 1 appears to be a major contributor to keratinocyte adhesion in skin. Studies have shown that autoantibodies to desmoglein 1 significantly enhance experimental acantholysis in animals treated with autoantibodies to desmoglein 3.
References
Amagai M, Tsunoda K, Zillikens D, Nagai T, Nishikawa T. The clinical phenotype of pemphigus is defined by the anti-desmoglein autoantibody profile. J Am Acad Dermatol. 1999;40(2 Pt 1):167-170.
Ding X, Aoki V, Mascaro JM Jr, Lopez-Swiderski A, Diaz LA, Fairley JA. Mucosal and mucocutaneous (generalized) pemphigus vulgaris show distinct autoantibody profiles. J Invest Dermatol. 1997;109(4):592-596.
Shirakata Y, Amagai M, Hanakawa Y, Nishikawa T, Hashimoto K. Lack of mucosal involvement in pemphigus foliaceus may be due to low expression of desmoglein 1. J Invest Dermatol. 1998;110(1):76-78.
Mahoney MG, Wang Z, Rothenberger K, Koch PJ, Amagai M, Stanley JR. Explanations for the clinical and microscopic localization of lesions in pemphigus foliaceus and vulgaris. J Clin Invest. 1999;103(4):461-468.
2. The structure labeled “e” is responsible for:
 c) Lamina densa-dermal adhesion
c) Lamina densa-dermal adhesion
The structure marked “e” is an anchoring fibril, which is the major fibrillar structure responsible for adhesion of the lamina densa to the underlying dermis. Other structures labeled in this Figure include collagen fibers (“c”), lamina densa (short broad arrowhead), hemidesmosome (white open arrowhead), lamina lucida (white asterisk), and sub-basal dense plate (long thin black arrow).
Anchoring fibrils are composed mainly, if not entirely, of aggregates of collagen VII dimers in antiparallel array that appear to arise within the lamina densa and insert either back into the lamina densa or into dermal structures called anchoring plaques. Other extracellular matrix proteins are also in these anchoring plaques. This arrangement produces an arcade of anchoring fibrils that interweave with dermal fibers to help firmly secure the lamina densa to the underlying dermis. This bond is extremely strong and is disrupted only in diseases associated with genetic defects in collagen VII (ie, dystrophic forms of epidermolysis bullosa) or autoimmunity to collagen VII (ie, epidermolysis bullosa acquista or bullous eruption of systemic lupus erythematosus).
Reference
Burgeson RE. Type VII collagen, anchoring fibrils, and epidermolysis bullosa. J Invest Dermatol. 1993;101(3):252-255.
3. Which of the following proteins is located within the region of the dermoepidermal junction indicated by the arrow?
 a) Laminin-332 (formerly named laminin-5)
a) Laminin-332 (formerly named laminin-5)
The region depicted by the arrow is the lamina lucida. Several proteins reside within or traverse a portion of that ultrastructural region, including laminin-1 and laminin-332, type XVII collagen (bullous pemphigoid antigen 2 [180-kD]), and uncein (an unconventional extracellular myosin). The ultrastructural region contains the anchoring filaments (wispy filaments, present exclusively underneath hemidesmosomes, which may be composed of laminin-332, laminin-6, uncein, and/or other proteins). In contrast, the hemidesmosome is composed of bullous pemphigoid antigen 1 (230-kD), the transmembrane portion of bullous pemphigoid antigen 2, plectin, and α6 β4 integrin. Keratins 5 and 14 comprise the tonofilaments which abut or attach into the cytoplasmic domain of the hemidesmosome. The lamina densa is composed of type IV collagen, heparin sulfate proteoglycan, chondroitin-6 sulfate proteoglycan, and nidogen/entactin. Within the sublamina densa and comprising the anchoring fibril, is type VII collagen. Types I and III collagen compose those collagen fibers which reside within the papillary and reticular dermis.
References
Mellerio JE. Molecular pathology of the cutaneous basement membrane zone. Clin Exp Dermatol. 1999;24(1):25-32.
Fine JD, McGrath J, Eady RA. Inherited epidermolysis bullosa comes into the new millenium: a revised classification system based on current knowledge of pathogenetic mechanisms and the clinical, laboratory, and epidemiologic findings of large, well-defined patient cohorts. J Am Acad Dermatol. 2000;43(1 Pt 1):135-137.
Jo-David Fine, MD, MPH, FRCP, is board certified in internal medicine, dermatology, and diagnostic and laboratory immunodermatology. Dr Fine served on the faculty of the Departments of Dermatology and Epidemiology in the Schools of Medicine and Public Health at the University of North Carolina at Chapel Hill.
Ronald J. Feldman, MD, PhD, is assistant professor of dermatology at Emory University Hospital in Atlanta, GA.
 1. This patient’s serum contains autoantibodies to desmoglein 3 and desmoglein 1. The diagnosis is:
1. This patient’s serum contains autoantibodies to desmoglein 3 and desmoglein 1. The diagnosis is:
a) Pemphigus vulgaris localized to mucous membranes
b) Generalized pemphigus vulgaris
c) Generalized pemphigus foliaceus
d) Mixed pemphigus vulgaris and pemphigus foliaceus
e) Paraneoplastic pemphigus
 2. The structure labeled “e” is responsible for:
2. The structure labeled “e” is responsible for:
a) Keratinocyte-keratinocyte adhesion
b) Keratinocyte-lamina densa adhesion
c) Lamina densa-dermal adhesion
d) Keratinocyte-dermal adhesion
e) Lamina densa-elastic fiber adhesion
 3. Which of the following proteins is located within the region of the dermoepidermal junction indicated by the arrow?
3. Which of the following proteins is located within the region of the dermoepidermal junction indicated by the arrow?
a) Laminin-332 (formerly named laminin-5)
b) Plectin
c) Keratin 5
d) Type IV collagen
e) Type VII collagen
Board Review Answers:
1. This patient’s serum contains autoantibodies to desmoglein 3 and desmoglein i.
 The diagnosis is:
The diagnosis is:
b) Generalized pemphigus vulgaris
Recent studies have provided an immunologic explanation (“desmoglein compensation theory”) for why some patients with pemphigus vulgaris have only mucosal lesions, while others have both mucosal and cutaneous lesions and why mucosal lesions do not occur in pemphigus foliaceus.
Pemphigus vulgaris patients with mucosal lesions, without significant skin lesions, have autoantibodies only to desmoglein 3. Pemphigus foliaceus patients lack mucosal involvement because they have autoantibodies only to desmoglein 1 and there is very little of that protein in the oral cavity. Coexistent desmoglein 3 stabilizes that tissue in pemphigus foliaceus. Desmoglein 3 is found on both mucosal and nonmucosal epithelial cells, but also appears to be the major cadhedrin keratinocyte adhesion protein on mucosal epithelial surfaces. Pemphigus vulgaris patients having both mucosal and skin lesions have autoantibodies to both desmoglein 3 and desmoglein 1. Desmoglein 1 appears to be a major contributor to keratinocyte adhesion in skin. Studies have shown that autoantibodies to desmoglein 1 significantly enhance experimental acantholysis in animals treated with autoantibodies to desmoglein 3.
References
Amagai M, Tsunoda K, Zillikens D, Nagai T, Nishikawa T. The clinical phenotype of pemphigus is defined by the anti-desmoglein autoantibody profile. J Am Acad Dermatol. 1999;40(2 Pt 1):167-170.
Ding X, Aoki V, Mascaro JM Jr, Lopez-Swiderski A, Diaz LA, Fairley JA. Mucosal and mucocutaneous (generalized) pemphigus vulgaris show distinct autoantibody profiles. J Invest Dermatol. 1997;109(4):592-596.
Shirakata Y, Amagai M, Hanakawa Y, Nishikawa T, Hashimoto K. Lack of mucosal involvement in pemphigus foliaceus may be due to low expression of desmoglein 1. J Invest Dermatol. 1998;110(1):76-78.
Mahoney MG, Wang Z, Rothenberger K, Koch PJ, Amagai M, Stanley JR. Explanations for the clinical and microscopic localization of lesions in pemphigus foliaceus and vulgaris. J Clin Invest. 1999;103(4):461-468.
2. The structure labeled “e” is responsible for:
 c) Lamina densa-dermal adhesion
c) Lamina densa-dermal adhesion
The structure marked “e” is an anchoring fibril, which is the major fibrillar structure responsible for adhesion of the lamina densa to the underlying dermis. Other structures labeled in this Figure include collagen fibers (“c”), lamina densa (short broad arrowhead), hemidesmosome (white open arrowhead), lamina lucida (white asterisk), and sub-basal dense plate (long thin black arrow).
Anchoring fibrils are composed mainly, if not entirely, of aggregates of collagen VII dimers in antiparallel array that appear to arise within the lamina densa and insert either back into the lamina densa or into dermal structures called anchoring plaques. Other extracellular matrix proteins are also in these anchoring plaques. This arrangement produces an arcade of anchoring fibrils that interweave with dermal fibers to help firmly secure the lamina densa to the underlying dermis. This bond is extremely strong and is disrupted only in diseases associated with genetic defects in collagen VII (ie, dystrophic forms of epidermolysis bullosa) or autoimmunity to collagen VII (ie, epidermolysis bullosa acquista or bullous eruption of systemic lupus erythematosus).
Reference
Burgeson RE. Type VII collagen, anchoring fibrils, and epidermolysis bullosa. J Invest Dermatol. 1993;101(3):252-255.
3. Which of the following proteins is located within the region of the dermoepidermal junction indicated by the arrow?
 a) Laminin-332 (formerly named laminin-5)
a) Laminin-332 (formerly named laminin-5)
The region depicted by the arrow is the lamina lucida. Several proteins reside within or traverse a portion of that ultrastructural region, including laminin-1 and laminin-332, type XVII collagen (bullous pemphigoid antigen 2 [180-kD]), and uncein (an unconventional extracellular myosin). The ultrastructural region contains the anchoring filaments (wispy filaments, present exclusively underneath hemidesmosomes, which may be composed of laminin-332, laminin-6, uncein, and/or other proteins). In contrast, the hemidesmosome is composed of bullous pemphigoid antigen 1 (230-kD), the transmembrane portion of bullous pemphigoid antigen 2, plectin, and α6 β4 integrin. Keratins 5 and 14 comprise the tonofilaments which abut or attach into the cytoplasmic domain of the hemidesmosome. The lamina densa is composed of type IV collagen, heparin sulfate proteoglycan, chondroitin-6 sulfate proteoglycan, and nidogen/entactin. Within the sublamina densa and comprising the anchoring fibril, is type VII collagen. Types I and III collagen compose those collagen fibers which reside within the papillary and reticular dermis.
References
Mellerio JE. Molecular pathology of the cutaneous basement membrane zone. Clin Exp Dermatol. 1999;24(1):25-32.
Fine JD, McGrath J, Eady RA. Inherited epidermolysis bullosa comes into the new millenium: a revised classification system based on current knowledge of pathogenetic mechanisms and the clinical, laboratory, and epidemiologic findings of large, well-defined patient cohorts. J Am Acad Dermatol. 2000;43(1 Pt 1):135-137.
Jo-David Fine, MD, MPH, FRCP, is board certified in internal medicine, dermatology, and diagnostic and laboratory immunodermatology. Dr Fine served on the faculty of the Departments of Dermatology and Epidemiology in the Schools of Medicine and Public Health at the University of North Carolina at Chapel Hill.
Ronald J. Feldman, MD, PhD, is assistant professor of dermatology at Emory University Hospital in Atlanta, GA.
 1. This patient’s serum contains autoantibodies to desmoglein 3 and desmoglein 1. The diagnosis is:
1. This patient’s serum contains autoantibodies to desmoglein 3 and desmoglein 1. The diagnosis is:
a) Pemphigus vulgaris localized to mucous membranes
b) Generalized pemphigus vulgaris
c) Generalized pemphigus foliaceus
d) Mixed pemphigus vulgaris and pemphigus foliaceus
e) Paraneoplastic pemphigus
 2. The structure labeled “e” is responsible for:
2. The structure labeled “e” is responsible for:
a) Keratinocyte-keratinocyte adhesion
b) Keratinocyte-lamina densa adhesion
c) Lamina densa-dermal adhesion
d) Keratinocyte-dermal adhesion
e) Lamina densa-elastic fiber adhesion
 3. Which of the following proteins is located within the region of the dermoepidermal junction indicated by the arrow?
3. Which of the following proteins is located within the region of the dermoepidermal junction indicated by the arrow?
a) Laminin-332 (formerly named laminin-5)
b) Plectin
c) Keratin 5
d) Type IV collagen
e) Type VII collagen
,
 1. This patient’s serum contains autoantibodies to desmoglein 3 and desmoglein 1. The diagnosis is:
1. This patient’s serum contains autoantibodies to desmoglein 3 and desmoglein 1. The diagnosis is:
a) Pemphigus vulgaris localized to mucous membranes
b) Generalized pemphigus vulgaris
c) Generalized pemphigus foliaceus
d) Mixed pemphigus vulgaris and pemphigus foliaceus
e) Paraneoplastic pemphigus
 2. The structure labeled “e” is responsible for:
2. The structure labeled “e” is responsible for:
a) Keratinocyte-keratinocyte adhesion
b) Keratinocyte-lamina densa adhesion
c) Lamina densa-dermal adhesion
d) Keratinocyte-dermal adhesion
e) Lamina densa-elastic fiber adhesion
 3. Which of the following proteins is located within the region of the dermoepidermal junction indicated by the arrow?
3. Which of the following proteins is located within the region of the dermoepidermal junction indicated by the arrow?
a) Laminin-332 (formerly named laminin-5)
b) Plectin
c) Keratin 5
d) Type IV collagen
e) Type VII collagen
To learn the answers, go to page 2
{{pagebreak}}
Board Review Answers:
1. This patient’s serum contains autoantibodies to desmoglein 3 and desmoglein i.
 The diagnosis is:
The diagnosis is:
b) Generalized pemphigus vulgaris
Recent studies have provided an immunologic explanation (“desmoglein compensation theory”) for why some patients with pemphigus vulgaris have only mucosal lesions, while others have both mucosal and cutaneous lesions and why mucosal lesions do not occur in pemphigus foliaceus.
Pemphigus vulgaris patients with mucosal lesions, without significant skin lesions, have autoantibodies only to desmoglein 3. Pemphigus foliaceus patients lack mucosal involvement because they have autoantibodies only to desmoglein 1 and there is very little of that protein in the oral cavity. Coexistent desmoglein 3 stabilizes that tissue in pemphigus foliaceus. Desmoglein 3 is found on both mucosal and nonmucosal epithelial cells, but also appears to be the major cadhedrin keratinocyte adhesion protein on mucosal epithelial surfaces. Pemphigus vulgaris patients having both mucosal and skin lesions have autoantibodies to both desmoglein 3 and desmoglein 1. Desmoglein 1 appears to be a major contributor to keratinocyte adhesion in skin. Studies have shown that autoantibodies to desmoglein 1 significantly enhance experimental acantholysis in animals treated with autoantibodies to desmoglein 3.
References
Amagai M, Tsunoda K, Zillikens D, Nagai T, Nishikawa T. The clinical phenotype of pemphigus is defined by the anti-desmoglein autoantibody profile. J Am Acad Dermatol. 1999;40(2 Pt 1):167-170.
Ding X, Aoki V, Mascaro JM Jr, Lopez-Swiderski A, Diaz LA, Fairley JA. Mucosal and mucocutaneous (generalized) pemphigus vulgaris show distinct autoantibody profiles. J Invest Dermatol. 1997;109(4):592-596.
Shirakata Y, Amagai M, Hanakawa Y, Nishikawa T, Hashimoto K. Lack of mucosal involvement in pemphigus foliaceus may be due to low expression of desmoglein 1. J Invest Dermatol. 1998;110(1):76-78.
Mahoney MG, Wang Z, Rothenberger K, Koch PJ, Amagai M, Stanley JR. Explanations for the clinical and microscopic localization of lesions in pemphigus foliaceus and vulgaris. J Clin Invest. 1999;103(4):461-468.
2. The structure labeled “e” is responsible for:
 c) Lamina densa-dermal adhesion
c) Lamina densa-dermal adhesion
The structure marked “e” is an anchoring fibril, which is the major fibrillar structure responsible for adhesion of the lamina densa to the underlying dermis. Other structures labeled in this Figure include collagen fibers (“c”), lamina densa (short broad arrowhead), hemidesmosome (white open arrowhead), lamina lucida (white asterisk), and sub-basal dense plate (long thin black arrow).
Anchoring fibrils are composed mainly, if not entirely, of aggregates of collagen VII dimers in antiparallel array that appear to arise within the lamina densa and insert either back into the lamina densa or into dermal structures called anchoring plaques. Other extracellular matrix proteins are also in these anchoring plaques. This arrangement produces an arcade of anchoring fibrils that interweave with dermal fibers to help firmly secure the lamina densa to the underlying dermis. This bond is extremely strong and is disrupted only in diseases associated with genetic defects in collagen VII (ie, dystrophic forms of epidermolysis bullosa) or autoimmunity to collagen VII (ie, epidermolysis bullosa acquista or bullous eruption of systemic lupus erythematosus).
Reference
Burgeson RE. Type VII collagen, anchoring fibrils, and epidermolysis bullosa. J Invest Dermatol. 1993;101(3):252-255.
3. Which of the following proteins is located within the region of the dermoepidermal junction indicated by the arrow?
 a) Laminin-332 (formerly named laminin-5)
a) Laminin-332 (formerly named laminin-5)
The region depicted by the arrow is the lamina lucida. Several proteins reside within or traverse a portion of that ultrastructural region, including laminin-1 and laminin-332, type XVII collagen (bullous pemphigoid antigen 2 [180-kD]), and uncein (an unconventional extracellular myosin). The ultrastructural region contains the anchoring filaments (wispy filaments, present exclusively underneath hemidesmosomes, which may be composed of laminin-332, laminin-6, uncein, and/or other proteins). In contrast, the hemidesmosome is composed of bullous pemphigoid antigen 1 (230-kD), the transmembrane portion of bullous pemphigoid antigen 2, plectin, and α6 β4 integrin. Keratins 5 and 14 comprise the tonofilaments which abut or attach into the cytoplasmic domain of the hemidesmosome. The lamina densa is composed of type IV collagen, heparin sulfate proteoglycan, chondroitin-6 sulfate proteoglycan, and nidogen/entactin. Within the sublamina densa and comprising the anchoring fibril, is type VII collagen. Types I and III collagen compose those collagen fibers which reside within the papillary and reticular dermis.
References
Mellerio JE. Molecular pathology of the cutaneous basement membrane zone. Clin Exp Dermatol. 1999;24(1):25-32.
Fine JD, McGrath J, Eady RA. Inherited epidermolysis bullosa comes into the new millenium: a revised classification system based on current knowledge of pathogenetic mechanisms and the clinical, laboratory, and epidemiologic findings of large, well-defined patient cohorts. J Am Acad Dermatol. 2000;43(1 Pt 1):135-137.
Jo-David Fine, MD, MPH, FRCP, is board certified in internal medicine, dermatology, and diagnostic and laboratory immunodermatology. Dr Fine served on the faculty of the Departments of Dermatology and Epidemiology in the Schools of Medicine and Public Health at the University of North Carolina at Chapel Hill.
Ronald J. Feldman, MD, PhD, is assistant professor of dermatology at Emory University Hospital in Atlanta, GA.
 1. This patient’s serum contains autoantibodies to desmoglein 3 and desmoglein 1. The diagnosis is:
1. This patient’s serum contains autoantibodies to desmoglein 3 and desmoglein 1. The diagnosis is:
a) Pemphigus vulgaris localized to mucous membranes
b) Generalized pemphigus vulgaris
c) Generalized pemphigus foliaceus
d) Mixed pemphigus vulgaris and pemphigus foliaceus
e) Paraneoplastic pemphigus
 2. The structure labeled “e” is responsible for:
2. The structure labeled “e” is responsible for:
a) Keratinocyte-keratinocyte adhesion
b) Keratinocyte-lamina densa adhesion
c) Lamina densa-dermal adhesion
d) Keratinocyte-dermal adhesion
e) Lamina densa-elastic fiber adhesion
 3. Which of the following proteins is located within the region of the dermoepidermal junction indicated by the arrow?
3. Which of the following proteins is located within the region of the dermoepidermal junction indicated by the arrow?
a) Laminin-332 (formerly named laminin-5)
b) Plectin
c) Keratin 5
d) Type IV collagen
e) Type VII collagen
Board Review Answers:
1. This patient’s serum contains autoantibodies to desmoglein 3 and desmoglein i.
 The diagnosis is:
The diagnosis is:
b) Generalized pemphigus vulgaris
Recent studies have provided an immunologic explanation (“desmoglein compensation theory”) for why some patients with pemphigus vulgaris have only mucosal lesions, while others have both mucosal and cutaneous lesions and why mucosal lesions do not occur in pemphigus foliaceus.
Pemphigus vulgaris patients with mucosal lesions, without significant skin lesions, have autoantibodies only to desmoglein 3. Pemphigus foliaceus patients lack mucosal involvement because they have autoantibodies only to desmoglein 1 and there is very little of that protein in the oral cavity. Coexistent desmoglein 3 stabilizes that tissue in pemphigus foliaceus. Desmoglein 3 is found on both mucosal and nonmucosal epithelial cells, but also appears to be the major cadhedrin keratinocyte adhesion protein on mucosal epithelial surfaces. Pemphigus vulgaris patients having both mucosal and skin lesions have autoantibodies to both desmoglein 3 and desmoglein 1. Desmoglein 1 appears to be a major contributor to keratinocyte adhesion in skin. Studies have shown that autoantibodies to desmoglein 1 significantly enhance experimental acantholysis in animals treated with autoantibodies to desmoglein 3.
References
Amagai M, Tsunoda K, Zillikens D, Nagai T, Nishikawa T. The clinical phenotype of pemphigus is defined by the anti-desmoglein autoantibody profile. J Am Acad Dermatol. 1999;40(2 Pt 1):167-170.
Ding X, Aoki V, Mascaro JM Jr, Lopez-Swiderski A, Diaz LA, Fairley JA. Mucosal and mucocutaneous (generalized) pemphigus vulgaris show distinct autoantibody profiles. J Invest Dermatol. 1997;109(4):592-596.
Shirakata Y, Amagai M, Hanakawa Y, Nishikawa T, Hashimoto K. Lack of mucosal involvement in pemphigus foliaceus may be due to low expression of desmoglein 1. J Invest Dermatol. 1998;110(1):76-78.
Mahoney MG, Wang Z, Rothenberger K, Koch PJ, Amagai M, Stanley JR. Explanations for the clinical and microscopic localization of lesions in pemphigus foliaceus and vulgaris. J Clin Invest. 1999;103(4):461-468.
2. The structure labeled “e” is responsible for:
 c) Lamina densa-dermal adhesion
c) Lamina densa-dermal adhesion
The structure marked “e” is an anchoring fibril, which is the major fibrillar structure responsible for adhesion of the lamina densa to the underlying dermis. Other structures labeled in this Figure include collagen fibers (“c”), lamina densa (short broad arrowhead), hemidesmosome (white open arrowhead), lamina lucida (white asterisk), and sub-basal dense plate (long thin black arrow).
Anchoring fibrils are composed mainly, if not entirely, of aggregates of collagen VII dimers in antiparallel array that appear to arise within the lamina densa and insert either back into the lamina densa or into dermal structures called anchoring plaques. Other extracellular matrix proteins are also in these anchoring plaques. This arrangement produces an arcade of anchoring fibrils that interweave with dermal fibers to help firmly secure the lamina densa to the underlying dermis. This bond is extremely strong and is disrupted only in diseases associated with genetic defects in collagen VII (ie, dystrophic forms of epidermolysis bullosa) or autoimmunity to collagen VII (ie, epidermolysis bullosa acquista or bullous eruption of systemic lupus erythematosus).
Reference
Burgeson RE. Type VII collagen, anchoring fibrils, and epidermolysis bullosa. J Invest Dermatol. 1993;101(3):252-255.
3. Which of the following proteins is located within the region of the dermoepidermal junction indicated by the arrow?
 a) Laminin-332 (formerly named laminin-5)
a) Laminin-332 (formerly named laminin-5)
The region depicted by the arrow is the lamina lucida. Several proteins reside within or traverse a portion of that ultrastructural region, including laminin-1 and laminin-332, type XVII collagen (bullous pemphigoid antigen 2 [180-kD]), and uncein (an unconventional extracellular myosin). The ultrastructural region contains the anchoring filaments (wispy filaments, present exclusively underneath hemidesmosomes, which may be composed of laminin-332, laminin-6, uncein, and/or other proteins). In contrast, the hemidesmosome is composed of bullous pemphigoid antigen 1 (230-kD), the transmembrane portion of bullous pemphigoid antigen 2, plectin, and α6 β4 integrin. Keratins 5 and 14 comprise the tonofilaments which abut or attach into the cytoplasmic domain of the hemidesmosome. The lamina densa is composed of type IV collagen, heparin sulfate proteoglycan, chondroitin-6 sulfate proteoglycan, and nidogen/entactin. Within the sublamina densa and comprising the anchoring fibril, is type VII collagen. Types I and III collagen compose those collagen fibers which reside within the papillary and reticular dermis.
References
Mellerio JE. Molecular pathology of the cutaneous basement membrane zone. Clin Exp Dermatol. 1999;24(1):25-32.
Fine JD, McGrath J, Eady RA. Inherited epidermolysis bullosa comes into the new millenium: a revised classification system based on current knowledge of pathogenetic mechanisms and the clinical, laboratory, and epidemiologic findings of large, well-defined patient cohorts. J Am Acad Dermatol. 2000;43(1 Pt 1):135-137.
Jo-David Fine, MD, MPH, FRCP, is board certified in internal medicine, dermatology, and diagnostic and laboratory immunodermatology. Dr Fine served on the faculty of the Departments of Dermatology and Epidemiology in the Schools of Medicine and Public Health at the University of North Carolina at Chapel Hill.
Ronald J. Feldman, MD, PhD, is assistant professor of dermatology at Emory University Hospital in Atlanta, GA.
Board Review Answers:
1. This patient’s serum contains autoantibodies to desmoglein 3 and desmoglein i.
 The diagnosis is:
The diagnosis is:
b) Generalized pemphigus vulgaris
Recent studies have provided an immunologic explanation (“desmoglein compensation theory”) for why some patients with pemphigus vulgaris have only mucosal lesions, while others have both mucosal and cutaneous lesions and why mucosal lesions do not occur in pemphigus foliaceus.
Pemphigus vulgaris patients with mucosal lesions, without significant skin lesions, have autoantibodies only to desmoglein 3. Pemphigus foliaceus patients lack mucosal involvement because they have autoantibodies only to desmoglein 1 and there is very little of that protein in the oral cavity. Coexistent desmoglein 3 stabilizes that tissue in pemphigus foliaceus. Desmoglein 3 is found on both mucosal and nonmucosal epithelial cells, but also appears to be the major cadhedrin keratinocyte adhesion protein on mucosal epithelial surfaces. Pemphigus vulgaris patients having both mucosal and skin lesions have autoantibodies to both desmoglein 3 and desmoglein 1. Desmoglein 1 appears to be a major contributor to keratinocyte adhesion in skin. Studies have shown that autoantibodies to desmoglein 1 significantly enhance experimental acantholysis in animals treated with autoantibodies to desmoglein 3.
References
Amagai M, Tsunoda K, Zillikens D, Nagai T, Nishikawa T. The clinical phenotype of pemphigus is defined by the anti-desmoglein autoantibody profile. J Am Acad Dermatol. 1999;40(2 Pt 1):167-170.
Ding X, Aoki V, Mascaro JM Jr, Lopez-Swiderski A, Diaz LA, Fairley JA. Mucosal and mucocutaneous (generalized) pemphigus vulgaris show distinct autoantibody profiles. J Invest Dermatol. 1997;109(4):592-596.
Shirakata Y, Amagai M, Hanakawa Y, Nishikawa T, Hashimoto K. Lack of mucosal involvement in pemphigus foliaceus may be due to low expression of desmoglein 1. J Invest Dermatol. 1998;110(1):76-78.
Mahoney MG, Wang Z, Rothenberger K, Koch PJ, Amagai M, Stanley JR. Explanations for the clinical and microscopic localization of lesions in pemphigus foliaceus and vulgaris. J Clin Invest. 1999;103(4):461-468.
2. The structure labeled “e” is responsible for:
 c) Lamina densa-dermal adhesion
c) Lamina densa-dermal adhesion
The structure marked “e” is an anchoring fibril, which is the major fibrillar structure responsible for adhesion of the lamina densa to the underlying dermis. Other structures labeled in this Figure include collagen fibers (“c”), lamina densa (short broad arrowhead), hemidesmosome (white open arrowhead), lamina lucida (white asterisk), and sub-basal dense plate (long thin black arrow).
Anchoring fibrils are composed mainly, if not entirely, of aggregates of collagen VII dimers in antiparallel array that appear to arise within the lamina densa and insert either back into the lamina densa or into dermal structures called anchoring plaques. Other extracellular matrix proteins are also in these anchoring plaques. This arrangement produces an arcade of anchoring fibrils that interweave with dermal fibers to help firmly secure the lamina densa to the underlying dermis. This bond is extremely strong and is disrupted only in diseases associated with genetic defects in collagen VII (ie, dystrophic forms of epidermolysis bullosa) or autoimmunity to collagen VII (ie, epidermolysis bullosa acquista or bullous eruption of systemic lupus erythematosus).
Reference
Burgeson RE. Type VII collagen, anchoring fibrils, and epidermolysis bullosa. J Invest Dermatol. 1993;101(3):252-255.
3. Which of the following proteins is located within the region of the dermoepidermal junction indicated by the arrow?
 a) Laminin-332 (formerly named laminin-5)
a) Laminin-332 (formerly named laminin-5)
The region depicted by the arrow is the lamina lucida. Several proteins reside within or traverse a portion of that ultrastructural region, including laminin-1 and laminin-332, type XVII collagen (bullous pemphigoid antigen 2 [180-kD]), and uncein (an unconventional extracellular myosin). The ultrastructural region contains the anchoring filaments (wispy filaments, present exclusively underneath hemidesmosomes, which may be composed of laminin-332, laminin-6, uncein, and/or other proteins). In contrast, the hemidesmosome is composed of bullous pemphigoid antigen 1 (230-kD), the transmembrane portion of bullous pemphigoid antigen 2, plectin, and α6 β4 integrin. Keratins 5 and 14 comprise the tonofilaments which abut or attach into the cytoplasmic domain of the hemidesmosome. The lamina densa is composed of type IV collagen, heparin sulfate proteoglycan, chondroitin-6 sulfate proteoglycan, and nidogen/entactin. Within the sublamina densa and comprising the anchoring fibril, is type VII collagen. Types I and III collagen compose those collagen fibers which reside within the papillary and reticular dermis.
References
Mellerio JE. Molecular pathology of the cutaneous basement membrane zone. Clin Exp Dermatol. 1999;24(1):25-32.
Fine JD, McGrath J, Eady RA. Inherited epidermolysis bullosa comes into the new millenium: a revised classification system based on current knowledge of pathogenetic mechanisms and the clinical, laboratory, and epidemiologic findings of large, well-defined patient cohorts. J Am Acad Dermatol. 2000;43(1 Pt 1):135-137.
Jo-David Fine, MD, MPH, FRCP, is board certified in internal medicine, dermatology, and diagnostic and laboratory immunodermatology. Dr Fine served on the faculty of the Departments of Dermatology and Epidemiology in the Schools of Medicine and Public Health at the University of North Carolina at Chapel Hill.
Ronald J. Feldman, MD, PhD, is assistant professor of dermatology at Emory University Hospital in Atlanta, GA.
 1. This patient’s serum contains autoantibodies to desmoglein 3 and desmoglein 1. The diagnosis is:
1. This patient’s serum contains autoantibodies to desmoglein 3 and desmoglein 1. The diagnosis is: 2. The structure labeled “e” is responsible for:
2. The structure labeled “e” is responsible for: 3. Which of the following proteins is located within the region of the dermoepidermal junction indicated by the arrow?
3. Which of the following proteins is located within the region of the dermoepidermal junction indicated by the arrow?





 1. This patient’s serum contains autoantibodies to desmoglein 3 and desmoglein 1. The diagnosis is:
1. This patient’s serum contains autoantibodies to desmoglein 3 and desmoglein 1. The diagnosis is: 2. The structure labeled “e” is responsible for:
2. The structure labeled “e” is responsible for: 3. Which of the following proteins is located within the region of the dermoepidermal junction indicated by the arrow?
3. Which of the following proteins is located within the region of the dermoepidermal junction indicated by the arrow? The diagnosis is:
The diagnosis is: The diagnosis is:
The diagnosis is:














