The feature “Calming the Cytokine Storm: The Potential Role of JAK Inhibitors in Treating COVID-19” in the May 2020 issue of The Dermatologist incorrectly stated Dr King’s disclosures and had numerous grammatical errors. These were corrected in the online version of the article on May 13, 2020.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the RNA virus causing the coronavirus disease (COVID-19) pandemic that has rapidly spread around the globe and already caused more than 1,000,000 documented infections in the United States alone. It has become clear that some patients with COVID-19 develop a cytokine release syndrome (CRS) and this may relate to poor outcome. IL-6 blockade with tocilizumab (Actemra) or sarilumab (Kevzara) is being tested for the treatment of COVID-19-associated CRS, given the utility of IL-6 blockade in CRS associated with chimeric antigen receptor (CAR) T cell therapy. However, other cytokines such as IL-2, interferon (IFN)-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), and granulocyte-colony stimulating factor (G-CSF) are also elevated in some of these patients.
Course of COVID-19 Infection
SARS-CoV-2 infects host cells through binding of the viral envelope spike (S) glycoprotein to the host cell receptor, angiotensin converting enzyme 2 (ACE2). ACE2 is expressed in the lungs, heart, kidneys, gastrointestinal tract, and vascular endothelium. Broadly speaking, the mechanisms of host injury due to SARS-CoV-2 are:
- Injury due to infection of the virus (eg, upper respiratory tract infection);
- Coagulopathy (eg, disseminated intravascular coagulation); and
- Immune injury (eg, cytokine release syndrome).
Usually, COVID-19 is an upper respiratory tract infection but it may progress to involve the lower respiratory tract, causing pneumonia. In the latter case, emerging evidence suggests that patients who develop more severe disease or die from SARS-CoV-2 infection have two phases of disease.1 Phase I roughly corresponds to the first week of symptoms and is characterized by high viral load and relatively mild symptoms (Figure 1). In phase II, which typically begins 9 to 12 days after symptom onset,1 patients develop more significant respiratory symptoms and potentially other end-organ damage. Acute respiratory distress syndrome (ARDS) typically occurs during this stage. Direct myocardial injury can be seen in about 25% of patients with severe disease.2 A coagulopathy, which may be profound and is poorly understood, may develop. It is thought that viral load during the late phase is lower than in the early phase.
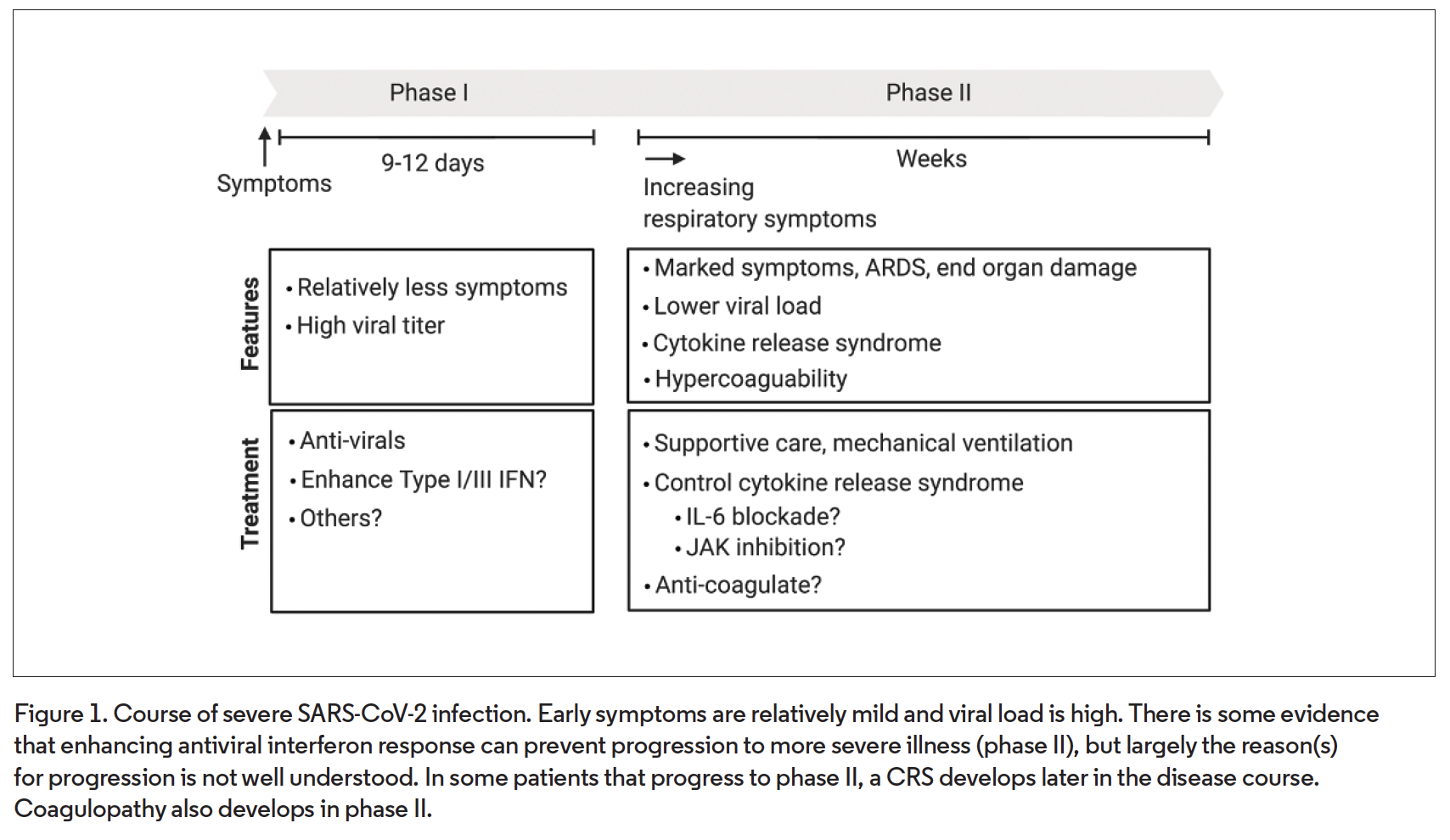
Although the reason for progression in some patients, but not others, is unclear, there is evidence that progression to the late stage is associated with, and possibly driven by, a CRS, as opposed to a direct effect of the virus itself.3 Why this happens is unclear, but the leading hypothesis is that a deficient early type I/III interferon response during phase I, which may be more likely in elderly patients or those with medical comorbidities, somehow paradoxically predisposes to hypercytokinemia during phase II. It has been hypothesized that mitigation of CRS could reduce morbidity and mortality from COVID-19.
Characteristics of CRS in COVID-19 and Treatment with IL-6 Blockade
The immunologic mechanism(s) leading to COVID-19-associated CRS are unclear. Laboratory findings in patients with COVID-19-associated CRS include lymphopenia (T cells in particular), elevated C-reactive protein (CRP), ferritin, lactate dehydrogenase (LDH), D-dimer, and pro-calcitonin.3-5 Studies have suggested that elevated plasma IL-6 levels may be associated with both COVID-19 severity and mortality.5,6 Several small studies have shown that levels of circulating IL-2, IL-7, IFN-γ, GM-CSF, G-CSF, and other cytokines are also elevated in these patients.7,8 Chemokines such as CXCL10 (T cells) and monocyte chemokines are also elevated. It is currently not understood if or how CRS is related to the hypercoagulability that develops in some critically ill COVID-19 patients. Mechanistic evaluation of potential links between CRS and the coagulopathy will be informative.
IL-6 blockade has emerged as a potentially promising approach to control COVID-19-associated CRS. There are two FDA-approved IL-6 receptor (IL-6Rα) blockers, tocilizumab and sarilumab. IL-6R blockade holds a theoretical advantage over IL-6 blockers (ie, siltuximab), given its broader activity against different forms of IL-6 signaling. Tocilizumab is FDA approved for the treatment of CRS induced by CAR-T cell cancer therapy, and so this may have been part of the rationale to initially try this drug in COVID-19-associated CRS. Clinical experience with IL-6 blockade in COVID-19 is increasing, and studies to evaluate the efficacy of this therapeutic modality are ongoing. Studies evaluating the utility of blocking the IL-1 signaling axis with drugs such as anakinra (Kineret; IL-1 receptor alpha blocker) are also occurring. In contrast to IL-6, IL-1 is not a JAK- signal transducer and activator of transcription (STAT) dependent cytokine.
Activity Against COVID-19-associated CRS
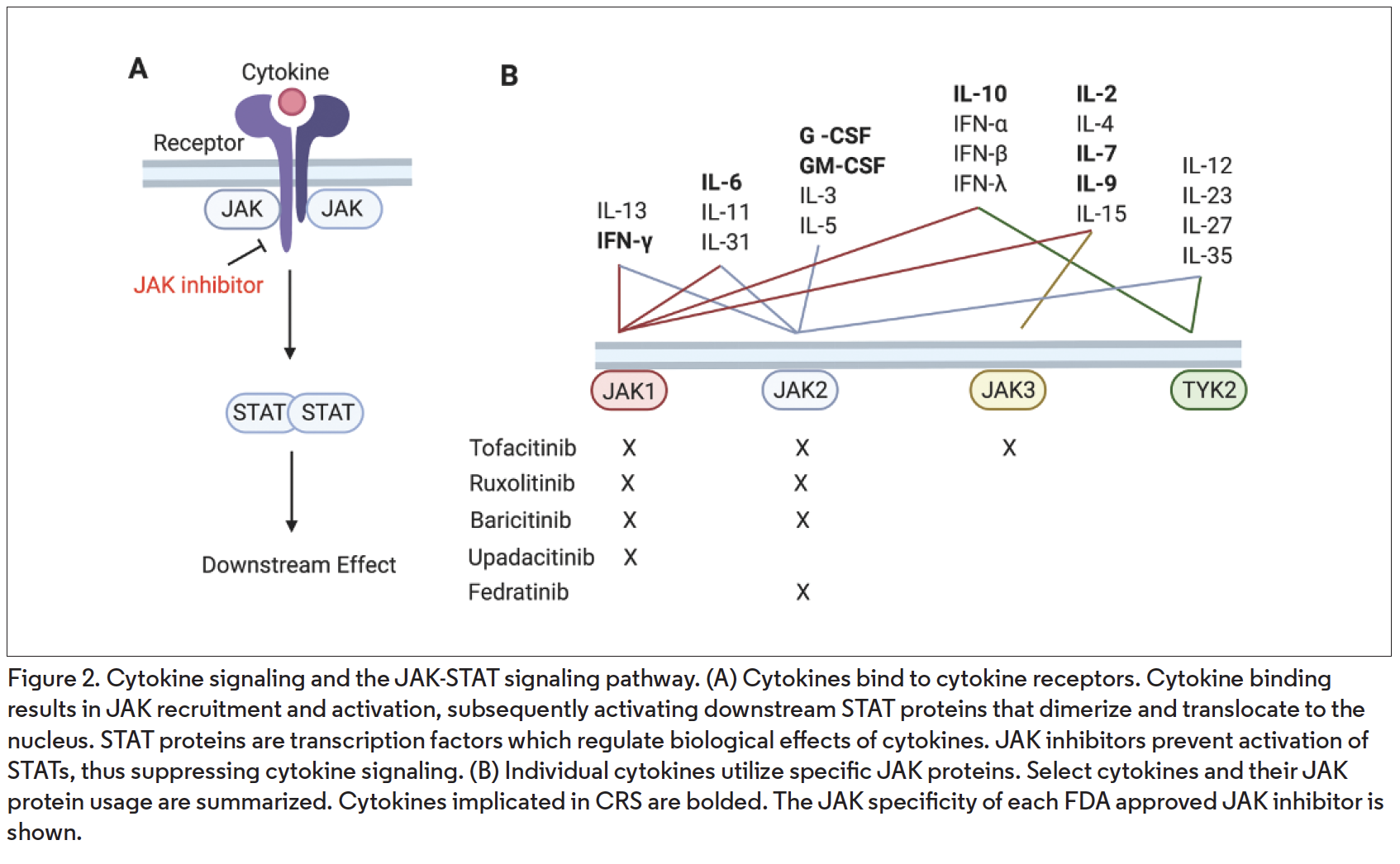
An alternate strategy for treating CRS is JAK blockade. JAK inhibitors are orally administered medications that inhibit the activity of cytokines that utilize the JAK-STAT pathway (Figure 2A), of which there are greater than 50.9 IL-6 is a JAK-STAT-dependent cytokine and its activity is blocked by JAK inhibitors.10 Compared with antibodies targeting a single cytokine or cytokine receptor, JAK inhibitors have the potential advantage of inhibiting the activity of multiple cytokines simultaneously. There are five FDA approved JAK inhibitors: tofacitinib (Xeljanz; JAK1/3>2, for rheumatoid arthritis, psoriatic arthritis, ulcerative colitis), ruxolitinib (Jakafi; JAK1/2, for myelofibrosis, polycythemia vera), baricitinib (Olumiant; JAK1/2, for rheumatoid arthritis), upadacitinib (Rinvoq; JAK1, for rheumatoid arthritis), and fedracitinib (Inrebic; JAK2, for myelofibrosis).
Interestingly, many cytokines implicated in COVID-19-associated CRS signal via the JAK-STAT pathway. These cytokines include IL-2, IL-6, IL-7, IL-10, G-CSF, GM-CSF, and IFN-γ (Figure 2B). Additionally, the expression of several chemokines that are elevated in COVID-19-associated CRS are regulated downstream of the above JAK-STAT dependent cytokines, and JAK inhibition suppresses their production in in vivo murine models.11,12
Of the JAK-STAT-dependent cytokines implicated in COVID-19, all but G-CSF and GM-CSF involve JAK1 (Figure 2B). G-CSF and GM-CSF signal via JAK2. Therefore, these data suggest that many of the FDA-approved JAK inhibitors may have efficacy in COVID-19-associated CRS.
Antiviral Immunity and JAK Inhibition
JAK proteins regulate signaling downstream of IFN-α/β (type I), IFN-γ (type II), and IFN-λ (type III), which are essential in antiviral immunity (Figure 2B). In light of this, it is interesting that rates of infectious events, including upper respiratory infections, nasopharyngitis, and influenza, are only mildly increased in JAK inhibitor-treated patients (compared to placebo) in clinical trials in various diseases.13 This seemingly mild impact on antiviral immunity may be because JAK inhibitors, which are administered orally one to two times daily and have short half-lives (hours), largely impact exaggerated cytokine activity, with relative sparing of normal cytokine activity, because drug concentrations are subtherapeutic for part of the day.14
Potential Treatment of Both COVID-19-associated CRS and Viral Infection
The potential role of JAK inhibitors in the treatment of COVID-19-associated CRS is clear. However, the ultimate treatment of COVID-19 might be an agent that is effective against both viral infection and the CRS induced by viral infection. It has been proposed that the JAK inhibitor baricitinib, by virtue of its unique chemical structure (not for its property as a JAK inhibitor), may have the ability to inhibit viral entry into cells. Computer modeling suggested that baricitinib might inhibit proteins potentially involved in SARS-CoV-2 entry into cells, including AP2-associated protein kinase 1 (AAK1).15 However, the concentration of baricitinib needed to inhibit AAK1 and clathrin-mediated endocytosis may require doses far above the FDA-approved dose of baricitinib.16 In any case, the theoretical effect against viral endocytosis only applies to baricitinib and is not a known property of other JAK inhibitors. Additional work will be needed to understand any possible clinical relevance of this observation.
Conclusions
The morbidity and mortality of COVID-19-associated CRS is significant, and effective treatments are direly needed. Given what is presently known, the potential for JAK inhibitor treatment of COVID-19-associated CRS is promising. Indeed, clinical trials with JAK inhibitors in hospitalized patients with moderate to severe disease are beginning. Despite the potential utility of this drug class in treating hospitalized patients with CRS, it recommended that any patient taking a JAK inhibitor that becomes infected with COVID-19 stop the medication. Most patients with COVID19 do not develop CRS and maintaining effective antiviral immune responses early in infection seems to be essential. Along these lines, treatment of nonhospitalized COVID-19 patients with mild-moderate disease with baricitinib (or continuation of baricitinib), for the (theoretical) purpose of inhibiting viral endocytosis (ie, early in infection), may not be advisable at this point due to the well-characterized activity of all JAK inhibitors in suppressing antiviral interferons. Needless to say, it will be informative to see how the activity of baricitinib compares to other JAK inhibitors in hospitalized COVID-19 patients. We are optimistic that these, and other studies, will provide novel insights into the pathobiology of this disease and help reduce both mortality and the burden on health care systems globally.
Dr Damsky is an instructor and dermatologist at the department of dermatology at Yale University School of Medicine in New Haven, CT. Dr King is an associate professor of dermatology at the Yale University School of Medicine.
Disclosure: Dr Damsky has research funding from Pfizer and has served as a consultant for Eli Lilly. Dr King has served on advisory boards or is a consultant for Aclaris Therapeutics Inc, Bristol-Meyers Squibb, Concert Pharmaceuticals Inc, Dermavant Sciences Inc, Eli Lilly and Company, and Pfizer Inc. He is on speakers bureau for Regeneron Pharmaceuticals, Sanofi Genzyme, and and Pfizer Inc.
References
1. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi:10.1016/S0140-6736(20)30566-3
2. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients With coronavirus disease 2019 (COVID-19). JAMA Cardiol. Published online March 27, 2020. doi:10.1001/jamacardio.2020.1017
3. Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130(5):2202-2205. doi:10.1172/JCI137647
4. Chen G, Wu D, Guo W, et al. Clinical and immunologic features in severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620-2629. doi:10.1172/JCI137244
5. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. Published online March 3, 2020. doi:10.1007/s00134-020-05991-x
6. Coomes EA, Haghbayan H. Interleukin-6 in COVID-19: a systematic review and meta-analysis. Preprint. medRxiv. Posted online April 3, 2020. doi:10.1101/2020.03.30.20048058
7. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi:10.1016/S0140-6736(20)30183-5
8. Yang Y, Shen C, Li J, et al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J Allergy Clin Immunol. Published online April 29, 2020. doi:10.1016/j.jaci.2020.04.027
9. Gadina M, Le MT, Schwartz DM, et al. Janus kinases to jakinibs: from basic insights to clinical practice. Rheumatology (Oxford). 2019;58(suppl 1):i4-i16. doi:10.1093/rheumatology/key432
10. Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting interleukin-6 signaling in clinic. Immunity. 2019;50(4):1007-1023. doi:10.1016/j.immuni.2019.03.026
11. Ghoreschi K, Jesson MI, Li X, et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J Immunol. 2011;186(7):4234-4243. doi:10.4049/jimmunol.1003668
12. LaBranche TP, Jesson MI, Radi ZA, et al. JAK inhibition with tofacitinib suppresses arthritic joint structural damage through decreased RANKL production. Arthritis Rheum. 2012;64(11):3531-3542. doi:10.1002/art.34649
13. Peterson D, Damsky W. King B. The use of Janus kinase inhibitors in the time of SARS-CoV-2. J Am Acad Dermatol. Published online April 9, 2020. doi:10.1016/j.jaad.2020.03.099
14. Clark JD, Flanagan ME, Telliez JB. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem. 2014;57(12):5023-5038. doi:10.1021/jm401490p
15. Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30-e31. doi:10.1016/S0140-6736(20)30304-4
16. Stebbing J, Phelan A, Griffin I, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20(4):400-402. doi:10.1016/S1473-3099(20)30132-8



























