Rotational Atherectomy for Calcified Coronary Lesions in Severe Aortic Stenosis Before Transcatheter Aortic Valve Implantation
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of the Journal of Invasive Cardiology or HMP Global, their employees, and affiliates.
J INVASIVE CARDIOL 2025. doi:10.25270/jic/25.00007. Epub April 4, 2025.
Abstract
Objectives. Calcified coronary artery disease (CAD) is prevalent in elderly patients with degenerative severe aortic stenosis (AS). Preparation of such calcified CAD using rotational atherectomy (RA) in those patients with severe AS is controversial and may carry a high risk of complications. The authors aimed to compare in-hospital outcomes following RA in patients with severe AS before transcatheter aortic valve implantation (TAVI) vs patients without AS.
Methods. The authors retrieved data from the Prospective Segeberg TAVI Registry from January 2016 to October 2021. All AS patients who underwent RA within 6 months prior to TAVI were included for our analysis and compared with patients without AS. In-hospital MACE, defined as cardiac mortality, myocardial infarction, and target lesion revascularization was evaluated in both groups.
Results. From a total of 472 patients who underwent RA, 38 (8.1%) patients had severe AS. The group with AS was older than the group without AS (84.4 ± 6.19 vs 75.2 ± 8.31; P < .001). About one-fourth of the RA procedures in the patients with AS were performed for aorto-ostial lesions (26.3%). Slow flow was reported in 1 (2.6%) patient in AS group, but no perforation or trapped burr was reported. In-hospital major adverse cardiovascular events (MACE) occurred in 41 (8.7%) patients and was comparable in both groups (7.9% in AS group vs 8.8% in non-AS group; P = .857). Furthermore, the presence of severe AS was not associated with occurrence of more in-hospital MACE following RA (OR 1.12: 95% CI, 0.33-3.81; P = .857). The cumulative rate of all-cause and cardiac mortality was higher in the AS group than in the non-AS group ((44.6% vs 22.2%, P = .002; 31.9% vs 17.2%, P = .017, respectively).
Conclusions. RA for preparing heavily calcified coronary lesions in patients with severe AS showed comparable in-hospital outcomes to patients without severe AS.
Introduction
Degenerative aortic stenosis (AS) is the most common valvular heart disease in western countries, particularly in elderly population, with a prevalence of about 10%.1 Because of shared risk factors, about 75% of patients with severe AS present with significant coronary artery disease (CAD).2 With the increasing number of elderly patients offered transcatheter aortic valve implantation (TAVI), the coexistence of severe AS and advanced coronary disease necessitates careful consideration of optimal therapeutic approaches to optimize patient outcomes. The risk of short-term mortality when performing percutaneous coronary intervention (PCI) is comparable in patients with severe AS and without AS.3
Rotational atherectomy (RA) is one of the plaque modification techniques used in the treatment of calcified CAD.4 While it has been studied and employed safely in patients with calcified coronary lesions, RA application in individuals with concomitant severe AS poses unique challenges and concerns.5,6
Controversies still exist around the timing and safety of RA escalation therapy in relation to managing calcified coronary lesions in patients with severe AS undergoing TAVI.6 RA could further compromise the inherent disturbance of coronary perfusion in AS and, thereby, result in myocardial injury as well as hemodynamic instability.7 The understanding of the clinical impact of RA before TAVI in this high-risk patient population is essential for guiding therapeutic decision making, optimizing procedure safety, and ultimately improving clinical outcomes.
In the current study, we reported the in-hospital clinical outcomes and periprocedural complications after RA in patients with severe AS before TAVI (AS group) compared with patients with no severe AS (non-AS group).
Methods
Study population
We retrieved data of all consecutively enrolled patients in The Prospective Segeberg TAVI Registry (ClinicalTrials.gov identifier: NCT03192774) from January 2016 to October 2021. All patients with AS who underwent RA prior to TAVI within a 6-month period were included for our analysis. During the study period, a total of 472 patients underwent RA. Of those, 38 (8.1%) patients had severe AS and received TAVI within 6 months after index RA, and the non-AS group included 434 patients.
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki. A written informed consent was obtained from all patients for analysis of their anonymized data, and data collection was approved by the local ethics committee.
Study objectives and definitions
We assessed the procedural safety of RA in patients with severe AS who were planned for TAVI within 6 months. In-hospital major adverse cardiovascular events (MACE) were defined as a composite of cardiac death, MI, and target lesion revascularization (TLR). Periprocedural MI (type 4a MI) was defined according to the fourth universal definition of MI.8 The target lesion was defined as the treated segment including the 5-mm margin proximal and distal to the stent. Periprocedural complications included coronary perforation, slow flow, trapped burr, and pericardial effusion and tamponade. Coronary perforation was defined and classified according to the Ellis classification.9 Slow flow was defined as a Thrombolysis in Myocardial Infarction (TIMI) flow grade of less than or equal to 2 at the end of the procedure.
Angiographic success was defined as stent implantation with a residual stenosis of less than 30% and TIMI-3 flow in the target vessel at the end of the procedure. Bail-out RA was defined as a procedure, where the decision to perform RA was made during the PCI after failure of balloon-based angioplasty. Elective RA was either pre- or intra-procedurally planned based on angiographic or intravascular imaging, but before any attempt of balloon angioplasty.
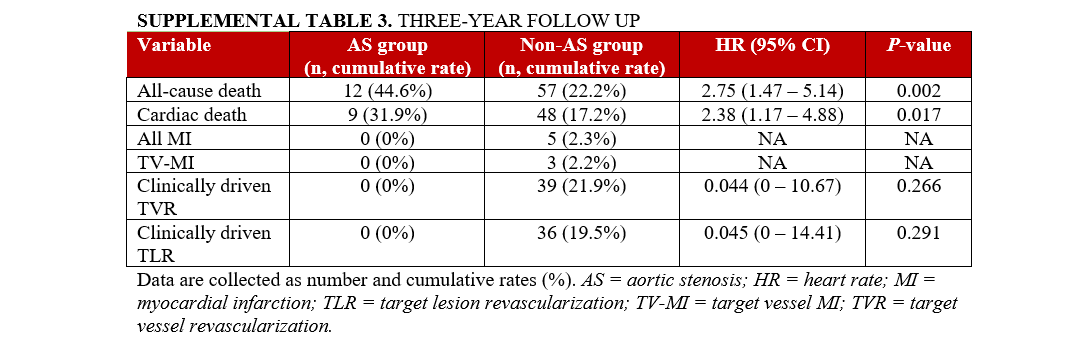
Long-term follow up was reported for 3 years. All-cause and cardiac mortality, MI, target-vessel MI, clinically driven target vessel revascularization (TVR), and clinically driven TLR were documented.
The main defining criterion for severe AS was a small aortic valve area (AVA), defined as less than 1 cm² (according to the European Society of Cardiology guidelines for the management of valvular heart diseases). Then, according to the transvalvular mean pressure gradient, the patients were dichotomized into high gradient (mean pressure gradient [MG] ≥ 40 mm Hg) and low gradient (MG < 40 mm Hg).10,11 For further evaluation, transesophageal echocardiography (TEE), multi-slice computed tomography, and, if needed, invasive hemodynamic measurements and dobutamine stress echocardiography for patients with low gradient and low left ventricular ejection fraction (LVEF), were performed.
Rotational atherectomy
RA was used in heavily calcified lesions according to the operator’s discretion and guided by an established institutional algorithm, previously described, as either an elective or a bail-out strategy.12 A procedure was regarded as an RA as soon as the ROTAWIRE was advanced through the target lesion. RA was performed using the Rotablator or ROTAPRO systems (Boston Scientific). Burr sizes of up to 0.7 of the vessel’s diameter were chosen. Oral aspirin of 325 to 500 mg and an oral loading dose of a P2Y12 inhibitor (clopidogrel in patients presenting with chronic coronary syndrome/prasugrel, or ticagrelor in patients presenting with acute coronary syndrome) was given prior to RA. Anticoagulation with either unfractionated heparin or bivalirudin was routinely administered during the procedure. There was no routine usage of a temporary pacemaker. Antiplatelet therapy regimen and duration was administered according to clinical presentation and as endorsed by guidelines.
Statistical analysis
Categorical variables were presented as frequencies and percentages, whereas continuous variables were expressed as mean ± SD or median (25th-75th quartiles), based on their distribution. To compare groups, the Student's t-test or Mann-Whitney U test was utilized for continuous variables, and the chi-square test was applied for categorical variables. When the chi-square test was unused because of small sample sizes, Fisher's exact test was used. All tests were 2-tailed, with a significance level set at a P-value of less than 0.05. Multivariate logistic regression analysis employed the enter method, incorporating covariates with P-values of less than 0.1 from the univariate analysis. Survival curves were constructed using the Kaplan–Meier method to assess the cumulative rates of all-cause and cardiac mortality, clinically driven TVR and TLR at 3 years, and the log-rank test was used to compare between the AS and non-AS groups. Data analysis was done using SPSS 24.0 software (IBM).
Results
Baseline patient characteristics
The mean age of the study population was 76 ± 8.45 years, and patients with severe AS were older that those without severe AS (84.4 ± 6.19 vs 75.2 ± 8.31; P < .001). The proportion of females in the AS group was higher compared with the non-AS group (39.5% vs 15.4%, P < .001). A total of 195 (41.3%) patients had undergone previous percutaneous coronary intervention (PCI), and 15.5% had a history of coronary artery bypass graft (CABG). A total of 292 (61.9%) patients had 3-vessel CAD, and more than half (57.9%) of the patients with AS had 3-vessel CAD. There was numerically more chronic kidney disease (CKD) in patients with AS than those without AS (36.8% vs 23.5%; P = .067). The clinical characteristics of both groups are summarized in Table 1. The data related to the characteristics of aortic stenosis in the AS group are listed in Supplemental Table 1.

Angiographic characteristics and procedural details
Almost all patients (n= 469 [99.4%]) had severely calcified coronary lesions (AHA B2/C). Patient without AS presented with more acute coronary syndromes (n = 1 [2.6%] vs n = 86 [19.8%], P < .009). About one-fourth of the RA procedures in patients with AS were performed for aorto-ostial lesions (n = 75 [26.3%] vs n = 10 [17.3%], P = .165). Elective RA was the main strategy for lesion preparation in both groups (n= 30 [78.9%] vs n = 300 [69.3%], P = 0.212), and smaller burr size was used in the non-AS group (1.5 [1.5-1.75] vs 1.5 [1.25-1.5]; P = .017). A total stent length of 60 mm or greater was implanted less in the AS group (n= 9 [23.7%] vs n= 184 [42.8%], P = .022). The total procedural duration was shorter in the AS group (75 [53-110] vs 95 [69-126] minutes; P = .016) and a lower amount of contrast medium was used (180 [130-247] vs 240 [176-320] mL; P = .001). The main angiographic and procedural characteristics of both groups are listed in Table 2.

Periprocedural complications and in-hospital outcomes
In-hospital MACE occurred in 41 (8.7%) patients and was comparable in both groups (n = 3, [7.9%] in the AS group vs n = 38 [8.8%] in the non-AS group; P = 0.857) (Figure). The rate of periprocedural complications was numerically higher in the non-AS group. The outcomes and complications are outlined in Table 3.


Using logistic regression analysis to determine the predictors for in-hospital MACE, we found that presence of severe AS was not associated with occurrence of more in-hospital MACE following RA (OR 1.12; 95% CI, 0.33-3.81); P = .857). The presence of CKD, hypertension, and low LVEF (≤ 35%) was associated with increased in-hospital MACE. Table 4 describes the uni- and multivariate logistic regression analysis.

The rate of cardiac mortality in the AS group was higher compared with the non-AS group (5.3% vs 2.5%, P = .324). Using logistic regression analysis to identify the factors associated with the numerical higher cardiac mortality, we identified the presence of hypertension, CKD, and low left ventricular systolic function as predictors. Predictors of in-hospital cardiac mortality are listed in Supplemental Table 2.
Long-term outcomes
Follow-up at 3 years was available in 84.7% (n = 399) of patients with a median of 310 days (IQR 42-740 days). The cumulative rate of all-cause and cardiac mortality was higher in the AS group than in the non-AS group (44.6% vs 22.2%, P = .002; 31.9% vs 17.2%, P = .017, respectively). The clinically driven TVR and TLR differed only numerically following 3 years between both groups. (Supplemental Table 3).
Discussion
The current study suggests that RA for the preparation of heavily calcified coronary lesions is procedurally safe in patients with severe AS. It reported a comparable rate of in-hospital MACE following RA in patients with severe AS and without AS. Additionally, the presence of severe AS before RA was not identified as a predictor of in-hospital MACE.
Patients with severe AS who were undergoing surgical aortic valve replacement (SAVR) had a high prevalence of 1 or more CAD, which increases with both age and the presence of valve calcification.13,14 A large Swedish registry showed that combined CABG and SAVR was performed most frequently in elderly patients, with a rate of 51.2% in patients aged 71 years or older.15 Furthermore, a study of 388 elderly patients (mean age 72 years) showed a strong association between aortic valve calcification and the presence of CAD in diagnostic coronary angiograms. These findings were also supported by a post-mortem study by Roberts et al that reported a presence of calcific deposit in the coronary arteries in 100% of patients with a calcific aortic valve.16 In the Post-TAVI registry from our center, which included about 1957 patients from 2007, almost half of patients with severe AS who underwent TAVI had two or more CAD (mean age 78.23 ± 5.6 years); this shows that aortic valve calcification can serve as a marker for atherosclerosis of the coronary arteries. The presence of aortic valve calcification, as well as mitral annular calcification (MAC), almost certainly arises from the same systemic vascular atherosclerotic process that leads to CAD.17
Our analysis of baseline patient characteristics revealed that patients with severe AS were older, highlighting the age-related nature of both severe AS and CAD. Additionally, there was a numerically higher prevalence of CKD in the group with severe AS, suggesting the presence of multiple comorbidities in this patient population.
Combined CABG and SAVR has been considered the standard therapeutic option for patients with severe AS and CAD. However, with the increasing age of the population, as well as the established TAVI concept as an alternative to SAVR in patients with intermediate and high surgical risk, the need for PCI is increasing. A large study by Goel et al showed that managing CAD with PCI in the presence of severe AS is feasible and safe, without an increased risk of short-term mortality compared with patients without AS.3 However, the safety of complex coronary intervention utilizing RA for treating heavily calcified CAD with the coexistence of severe AS with high probability of hemodynamic compromise is not clear. With the prevalence of such cases, further studies are needed to evaluate the safety of this complex intervention.18,19
Our study demonstrated comparable rates of in-hospital MACE between the severe AS and non-AS groups. Although the rate of cardiac mortality in the AS group was numerically higher, the identified predictors of this event were the presence of hypertension, CKD, and impaired left ventricular systolic function, not the presence of AS. Kotronias et al reported similar results of a numerically higher 30-day mortality rate that was not correlated to the presence of AS. Nevertheless, this study reported a significantly higher 1-year mortality because of a 5-fold increased hazard of death among patients with severe AS who did not proceed to aortic valve replacement.20 Long-term follow-up of our patients demonstrated a higher all-cause and cardiac mortality in the AS group, which is not surprising because of the presence of other comorbidities in older patients with AS. However, the rate of TVR and TLR was comparable in both groups.
Although the management of CAD with PCI in presence of severe AS proved to be feasible and safe, as previously mentioned, the timing of intervention is still questionable, particularly when a complex coronary intervention is required. A study of coronary intervention before TAVI showed an increased risk of bleeding, kidney injury, and stroke.21 On the other hand, incomplete revascularization increases long-term mortality following TAVI.22 Still, clinical guidelines advocate treatment of significant CAD before TAVI, with only a low level of evidence (class 2a, level C).23 A clinical consensus statement was published with granular guidance on performing PCI only in some clinical indications, and pending a generalized indication subject to the results of ongoing randomized controlled trials assessing invasive coronary physiology.24 One of these trials is the Notion-3 Study, which showed that coronary revascularization before TAVI resulted in a 29% lower relative risk of all-cause mortality, MI, or urgent revascularization after a median of 2 years compared with medical therapy before TAVI.25 Our analysis showed that the complexity of coronary lesions that mandated the use of plaque modification techniques such as RA was yet safe as a modality of therapy in patients with AS. In a retrospective analysis of 29 patients with severe AS who underwent elective RA, no clinically significant adverse events were reported, despite transient hemodynamic changes.26 A second retrospective analysis of 27 patients echoed the same results, indicating the feasibility and low rate of procedural complications in patients with AS.6 We included more patients with an advantageous comparison with patients without AS. The findings from our analysis advocate the established necessity of aggressive plaque modification techniques in calcified coronary lesions to achieve better outcomes without affecting safety of patients.5,27 Our study was not intended to question the timing of PCI in relation to TAVI as other studies have, which showed better outcomes when PCI was performed after TAVI.21
Limitations
Our results should be interpreted with caution given the number of patients and the single-center nature of the study. As RA is a technically challenging procedure, clinical outcomes may vary depending on the center’s experience. Our understanding and conclusions based on the results from the study might lack reproducibility but warrant further dedicated multicenter studies to prove applicability. Furthermore, these findings can inform future research and guide clinicians in providing the most appropriate treatment strategies for these challenging subsets of population.
Conclusions
In our older study population, coronary lesion preparation using RA in patients with severe AS before TAVI was feasible, and in-hospital events were comparable with patients without AS.
Affiliations and Disclosures
Sultan Alotaibi, MD, MSc1,2; Abdelhakim Allali, MD2,3; Hajo Heyer, MD2; Nader Mankerious, MD2,4; Martin Landt, MD2; Mohamed Abdel-Wahab, MD5; Volker Geist, MD2; Ralph Tölg, MD2,6,7; Mohamed Samy, MD2,4; Gert Richardt, MD2,6; Karim Elbasha, MD2,4
From the 1Cardiac Center, King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia; 2Cardiology Department, Heart Centre Segeberger Kliniken GmbH, Bad Segeberg, Germany; 3Department of Cardiology, Angiology and Intensive Care Medicine, Medical Clinic II, University Heart Center Lübeck, Lübeck, Germany; 4Cardiology department, Faculty of Medicine Zagazig University, Egypt; 5Cardiology Department, Heart Centre Leipzig at University of Leipzig, Leipzig, Germany; 6Center for Cardiovascular and Diabetes Medicine, Asklepios Clinic Bad Oldesloe, Bad Oldesloe, Germany; 7Medical Faculty of the Christian-Albrechts-University of Kiel, Kiel, Germany.
Acknowledgments: The authors gratefully acknowledge Susanne Sachse, Monika Bahnsen-Maass, Friederike Geyer, Daniela Schuermann-Kuchenbrandt and Wiebke Mohr-toedt for their assistance in data collection.
Disclosures: Dr Allali is consultant and proctor for Boston Scientific and a consultant for Shockwave Medical. Dr Richardt has received institutional research grants from Biotronik and Medtronic. The remaining authors report no financial relationships or conflicts of interest regarding the content herein.
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Address for correspondence: Sultan Alotaibi, MD, MSc, FESC, FSCAI, Cardiac Center
King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia. Email: Sultan.otb@gmail.com
X: @sultanotb
References
1. Fattouch K, Castrovinci S, Carità P. Aortic valve stenosis: treatments options in elderly high-risk patients. J Geriatr Cardiol. 2016;13(6):473-474. doi:10.11909/j.issn.1671-5411.2016.06.008
2. Goel SS, Ige M, Tuzcu EM, et al. Severe aortic stenosis and coronary artery disease--implications for management in the transcatheter aortic valve replacement era: a comprehensive review. J Am Coll Cardiol. 2013;62(1):1-10. doi:10.1016/j.jacc.2013.01.096
3. Goel SS, Agarwal S, Tuzcu EM, et al. Percutaneous coronary intervention in patients with severe aortic stenosis: implications for transcatheter aortic valve replacement. Circulation. 2012;125(8):1005-1013. doi:10.1161/CIRCULATIONAHA.111.039180
4. Fourrier JL, Bertrand ME, Auth DC, Lablanche JM, Gommeaux A, Brunetaud JM. Percutaneous coronary rotational angioplasty in humans: preliminary report. J Am Coll Cardiol. 1989;14(5):1278-1282. doi:10.1016/0735-1097(89)90428-2
5. Abdel-Wahab M, Richardt G, Joachim Büttner H, et al. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc Interv. 2013;6(1):10-19. doi:10.1016/j.jcin.2012.07.017
6. Bhatia KS, Sritharan HP, Allahwala U, Ward M, Bhindi R. Safety and feasibility of rotational atherectomy in severe aortic stenosis. Heart Lung Circ. 2022;31(5):666-670. doi:10.1016/j.hlc.2021.12.004
7. Mancusi C, Bahlmann E, Basile C, Gerdts E. New evidence about aortic valve stenosis and cardiovascular hemodynamics. High Blood Press Cardiovasc Prev. 2022;29(3):231-237. doi:10.1007/s40292-022-00520-x
8. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Glob Heart. 2012;7(4):275-295. doi:10.1016/j.gheart.2012.08.001
9. Ellis SG, Ajluni S, Arnold AZ, et al. Increased coronary perforation in the new device era. Incidence, classification, management, and outcome. Circulation. 1994;90(6):2725-2730. doi:10.1161/01.cir.90.6.2725
10. Baumgartner H, Falk V, Bax JJ, et al; ESC Scientific Document Group. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739-2791. doi:10.1093/eurheartj/ehx391
11. Vahanian A, Beyersdorf F, Praz F, et al; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. 2021;60(4):727-800. doi:10.1093/ejcts/ezab389
12. Allali A, Abdel-Wahab M, Elbasha K, et al. Rotational atherectomy of calcified coronary lesions: current practice and insights from two randomized trials. Clin Res Cardiol. 2023;112(9):1143-1163. doi:10.1007/s00392-022-02013-2
13. Rapp AH, Hillis LD, Lange RA, Cigarroa JE. Prevalence of coronary artery disease in patients with aortic stenosis with and without angina pectoris. Am J Cardiol. 2001;87(10):1216-1217; A7. doi:10.1016/s0002-9149(01)01501-6
14. Adler Y, Vaturi M, Herz I, et al. Nonobstructive aortic valve calcification: a window to significant coronary artery disease. Atherosclerosis. 2002;161(1):193-197. doi:10.1016/s0021-9150(01)00617-7
15. Kvidal P, Bergström R, Hörte LG, Ståhle E. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol. 2000;35(3):747-756. doi:10.1016/s0735-1097(99)00584-7
16. Roberts WC. Morphologic features of the normal and abnormal mitral valve. Am J Cardiol. 1983;51(6):1005-1028. doi:10.1016/s0002-9149(83)80181-7
17. Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29(3):630-634. doi:10.1016/s0735-1097(96)00563-3.
18. Paradis JM, White JM, Généreux P, et al. Impact of coronary artery disease severity assessed with the SYNTAX score on outcomes following transcatheter aortic valve replacement. J Am Heart Assoc. 2017;6(2):e005070. doi:10.1161/JAHA.116.005070
19. Witberg G, Regev E, Chen S, et al. The prognostic effects of coronary disease severity and completeness of revascularization on mortality in patients undergoing transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2017;10(14):1428-1435. doi:10.1016/j.jcin.2017.04.035
20. Kotronias RA, Scarsini R, Gibbs T, et al. Safety of rotational atherectomy using the radial access in patients with severe aortic stenosis. Am J Cardiol. 2019;124(3):381-388. doi:10.1016/j.amjcard.2019.04.052
21. Lunardi M, Venturi G, Del Sole PA, et al. Optimal timing for percutaneous coronary intervention in patients undergoing transcatheter aortic valve implantation. Int J Cardiol. 2022;365:114-122. doi:10.1016/j.ijcard.2022.07.030
22. Witberg G, Zusman O, Codner P, Assali A, Kornowski R. Impact of coronary artery revascularization completeness on outcomes of patients with coronary artery disease undergoing transcatheter aortic valve replacement: a meta-analysis of studies using the residual SYNTAX score (Synergy Between PCI With Taxus and Cardiac Surgery). Circ Cardiovasc Interv. 2018;11(3):e006000. doi:10.1161/CIRCINTERVENTIONS.117.006000
23. Vahanian A, Beyersdorf F, Praz F, et al; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561-632. doi:10.1093/eurheartj/ehab395
24. Tarantini G, Tang G, Nai Fovino L, et al. Management of coronary artery disease in patients undergoing transcatheter aortic valve implantation. A clinical consensus statement from the European Association of Percutaneous Cardiovascular Interventions in collaboration with the ESC Working Group on Cardiovascular Surgery. EuroIntervention. 2023;19(1):37-52. doi:10.4244/EIJ-D-22-00958
25. Lønborg J, Jabbari R, Sabbah M, et al. NOTION-3 Study Group. PCI in patients undergoing transcatheter aortic-valve implantation. N Engl J Med. 2024;391(23):2189-2200. doi:10.1056/NEJMoa2401513
26. Lippmann M, Patel J, Kvapil J, et al. Safety and feasibility of rotational atherectomy in elderly patients with severe aortic stenosis. J Invasive Cardiol. 2017;29(8):271-275.
27. Abdel-Wahab M, Toelg R, Byrne RA, et al. High-speed rotational atherectomy versus modified balloons prior to drug-eluting stent implantation in severely calcified coronary lesions. Circ Cardiovasc Interv. 2018;11(10):e007415. doi:10.1161/CIRCINTERVENTIONS.118.007415