Can IodixAnol ReducE the Incidence of Adverse Renal or Cardiac Events in Chronic Total Occlusion Interventions (CARE-CTO): A Substudy of the PROGRESS-CTO Registry
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of the Journal of Invasive Cardiology or HMP Global, their employees, and affiliates.
J INVASIVE CARDIOL 2025. doi:10.25270/jic/25.00004. Epub April 3, 2025.
Abstract
Objectives. The impact of contrast type in chronic total occlusion (CTO) percutaneous coronary intervention (PCI) remains controversial. The authors sought to evaluate the impact of contrast medium selection on patients undergoing CTO PCI.
Methods. The authors examined the outcomes of patients who underwent CTO PCI using iso-osmolar (iodixanol) vs pooled low-osmolar contrast media (LOCM) using data from the PROGRESS-CTO registry.
Results. Iodixanol was used in 1007 (18.1%) of 5558 CTO PCIs. Compared with pooled LOCM, iodixanol-treated patients were more likely to be women, were older, and more likely to have diabetes, dyslipidemia, hypertension, history of heart failure, myocardial infarction, coronary artery bypass graft surgery, and stroke. Iodixanol cases had higher complexity, with longer lesion length (35.25 ± 25.16 vs 28.91 ± 19.46 mm, P < .001), higher prevalence of moderate or severe calcification (43% vs 37%, P < .001) and moderate or severe proximal tortuosity (30% vs 24%, P < .001), and higher Japanese-CTO (2.52 vs 2.17, P < .001) and PROGRESS-CTO scores (1.30 vs 1.16, P < .001). Iodixanol cases required longer procedure times but similar contrast volumes. Technical (85.3% vs 89.2%, P = .001) and procedural success (83.4% vs 87.3%, P = .001) were lower in iodixanol cases. Acute kidney injury (AKI) occurred in 6.4% of cases. After propensity score matching, the patients who received iodixanol had lower incidence of AKI (odds ratio [OR]: 0.67; 95% CI, 0.47, 0.97; P = .032) and a trend for lower incidence of major adverse renal or cardiovascular events (OR: 0.75; 95% CI, 0.56, 1.0; P = .061).
Conclusions. AKI occurred in approximately 6% of CTO PCI cases. Iodixanol use was associated with a lower incidence of AKI, despite being used in more complex patients.
Introduction
Contrast-associated acute kidney injury (CA-AKI) is a known complication of percutaneous coronary intervention (PCI) and is more likely to occur with complex PCI, such as chronic total occlusion (CTO) PCI.1 Adequate hydration and minimizing contrast volume can help reduce the risk of CA-AKI, with some studies suggesting lower risk with iso-osmolar contrast media2 (Table 1). We examined the outcomes with iso-osmolar (iodixanol [Visipaque; GE HealthCare]) vs low-osmolar contrast agents in patients undergoing CTO PCI in a large multicenter registry.

Methods
We reviewed the baseline clinical and angiographic characteristics, along with the procedural outcomes, of 5558 CTO PCI procedures performed between 2012 and 2024 across 54 centers in the United States and internationally, with available data on contrast utilization. Data were collected using a dedicated online database (PROGRESS CTO: Prospective Global Registry for the Study of Chronic Total Occlusion Intervention; ClinicalTrials.gov identifier: NCT02061436). REDCap (Research Electronic Data Capture) hosted by the Minneapolis Heart Institute Foundation was used to record and manage data. The study received approval from the institutional review board at each participating center, and patient consent was waived.
Coronary CTOs were defined as coronary lesions with Thrombolysis in Myocardial Infarction (TIMI) grade 0 flow for at least 3 months. Occlusion duration was estimated based on clinical factors, such as the initial onset of angina, a history of myocardial infarction (MI) in the target vessel territory, or a comparison with previous angiographic findings.
Moderate proximal vessel tortuosity was defined as having at least 2 bends greater than 70° or 1 bend greater than 90°, while severe tortuosity was defined as having 2 bends greater than 90° or 1 bend greater than 120° in the CTO vessel. Calcification was evaluated by angiography and categorized as mild (spots), moderate (involving ≤ 50% of the reference lesion diameter), or severe (> 50%). Technical success was defined as the successful revascularization of the CTO with less than 30% residual stenosis in the treated segment and the restoration of TIMI grade 3 antegrade flow. Procedural success was defined as technical success without any in-hospital major adverse cardiac events (MACE). In-hospital MACE included any of the following adverse events prior to hospital discharge: death, myocardial infarction, recurrent symptoms requiring urgent repeat target-vessel revascularization with PCI or coronary artery bypass graft (CABG) surgery, tamponade requiring either pericardiocentesis or surgery, and stroke. MI was defined according to the Fourth Universal Definition of Myocardial Infarction (type 4a MI). The Japanese-CTO (J-CTO) score was calculated as described by Morino et al,3 the PROGRESS-CTO score according to Christopoulos et al,4 and the new PROGRESS-CTO complication scores (Acute MI, MACE, Mortality, and Pericardiocentesis) as described by Simsek et al.5
The primary endpoint of the study was AKI that was defined as an increase in serum creatinine by either ≥ 0.3 mg/dL (≥ 26.5 μmol/L) within 48 hours, or an increase in serum creatinine to 1.25 times the baseline after contrast administration and after other causes of renal impairment were excluded.6 This definition differs from the one suggested by the CTO academic research consortium (ARC).7 We used creatinine values only to calculate the KDIGO (Kidney Disease: Improving Global Outcomes) and ARC CTO AKI stages. Secondary endpoints included major adverse renal or cardiovascular events (MARCE, defined as the composite of death, MI, coronary revascularization, and AKI), dialysis, MI, stent thrombosis, transient ischemic attack or stroke, and death.
Categorical variables were presented as percentages and compared using the Pearson’s chi-square test. Continuous variables were expressed as mean ± SD or median (IQR), unless otherwise specified. Comparisons were made using the independent-samples t-test for normally distributed variables and the Mann-Whitney U test for non-parametric variables, as appropriate. Missing values for demographic and angiographic variables were imputed. Imputation was performed using predictive mean matching for continuous variables, logistic regression for binary variables, polytomous logistic regression for non-binary categorical variables, and proportional odds models for ordered categorical variables, as appropriate. We used R Statistical Software, version 4.4.0 (R Foundation for Statistical Computing) for all statistical analyses. A P-value of less than 0.05 was considered statistically significant.
Results
Of the 5558 CTO PCIs performed between 2012 to 2024, 1007 (18.1%) were performed using iodixanol (Figure 1). Patients who received iodixanol were older (mean age 66.7 vs 63.7 years, P < .001), more likely to be female (23.1% vs 20.2%, P = .041) and more likely to have comorbidities such as diabetes mellitus, hypertension, dyslipidemia, history of heart failure, PCI, MI, CABG, and stroke (Table 2).

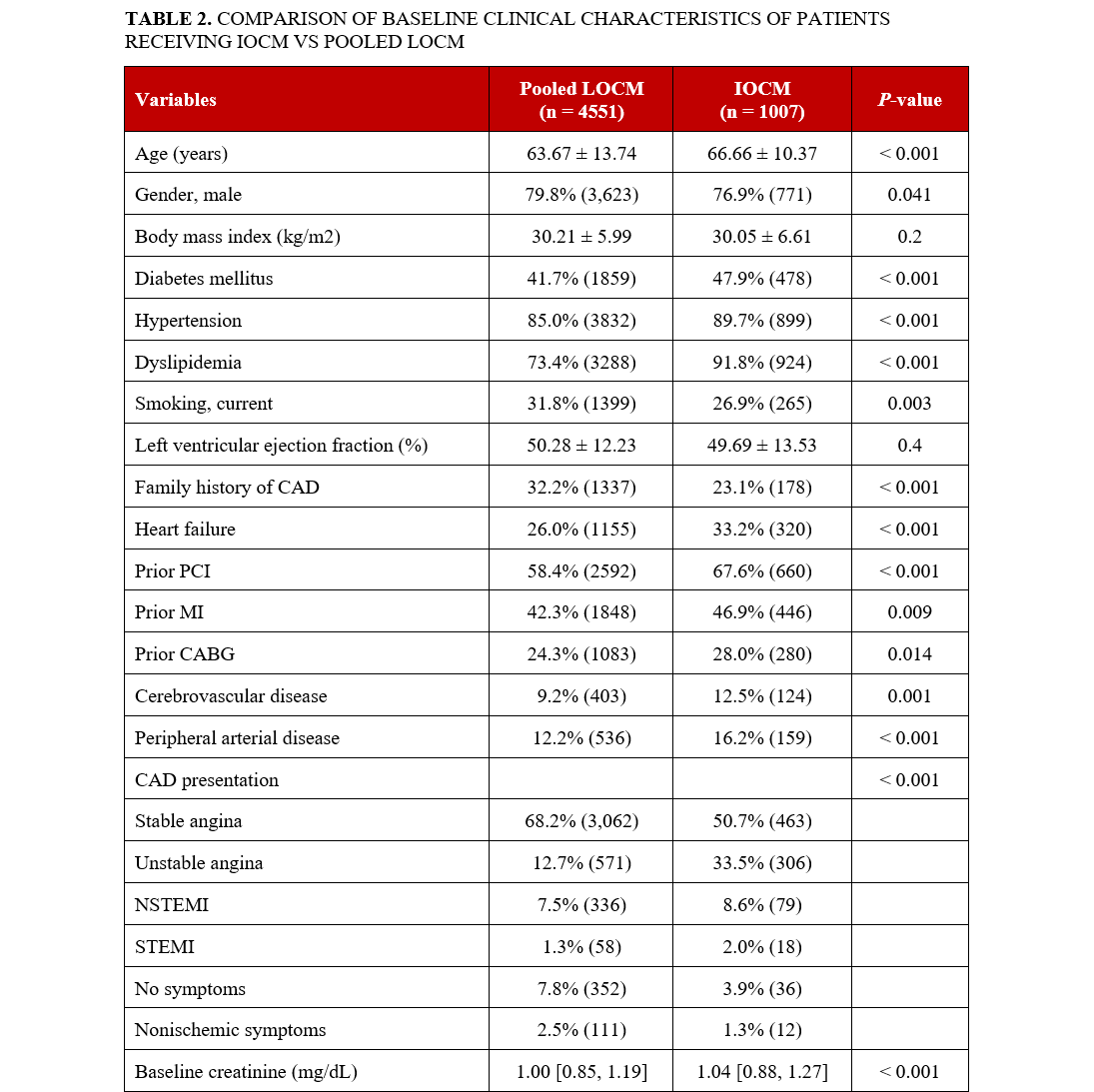

Iodixanol cases were more complex, with longer lesion length (35.3 vs 28.9 mm, P < .001), higher prevalence of moderate or severe calcification (43.1% vs 37.2%, P < .001) and moderate or severe proximal vessel tortuosity (30.1% vs 24.4%, P < .001), higher J-CTO (2.52 vs 2.17, P < .001) and PROGRESS-CTO (1.30 vs 1.16, P < .001) scores, and lower prevalence of interventional collaterals (43.8% vs 60.5%, P < .001) (Table 3).

Iodixanol lesions were more likely to be treated using femoral access (85.6% vs 68.5%, P < .001) and to be balloon uncrossable (12.2% vs 7.9%, P < .001) and balloon undilatable (7.0% vs 4.9%, P = .009) (Table 4). They required longer procedure times (118.0 vs 103.0, P < .001), similar contrast volume (204 vs 200 ml, P = .600), and shorter fluoroscopy times (38.8 vs 41.8 minutes, P = .002).

Iodixanol cases had lower technical (85.2% vs 89.2%, P = .001) and procedural success (83.4% vs 87.3%, P = .001). After adjusting for confounders, contrast type was not associated with technical success (odds ratio [OR]: 0.815; 95% CI, 0.644, 1.032; P = .090), procedural success (0.867; 95% CI, 0.676, 1.112; P = .224), MARCE (7.4% vs 8.6%, P = .253), or MACE (2.9% vs 2.3%, P = .361) (Table 5).


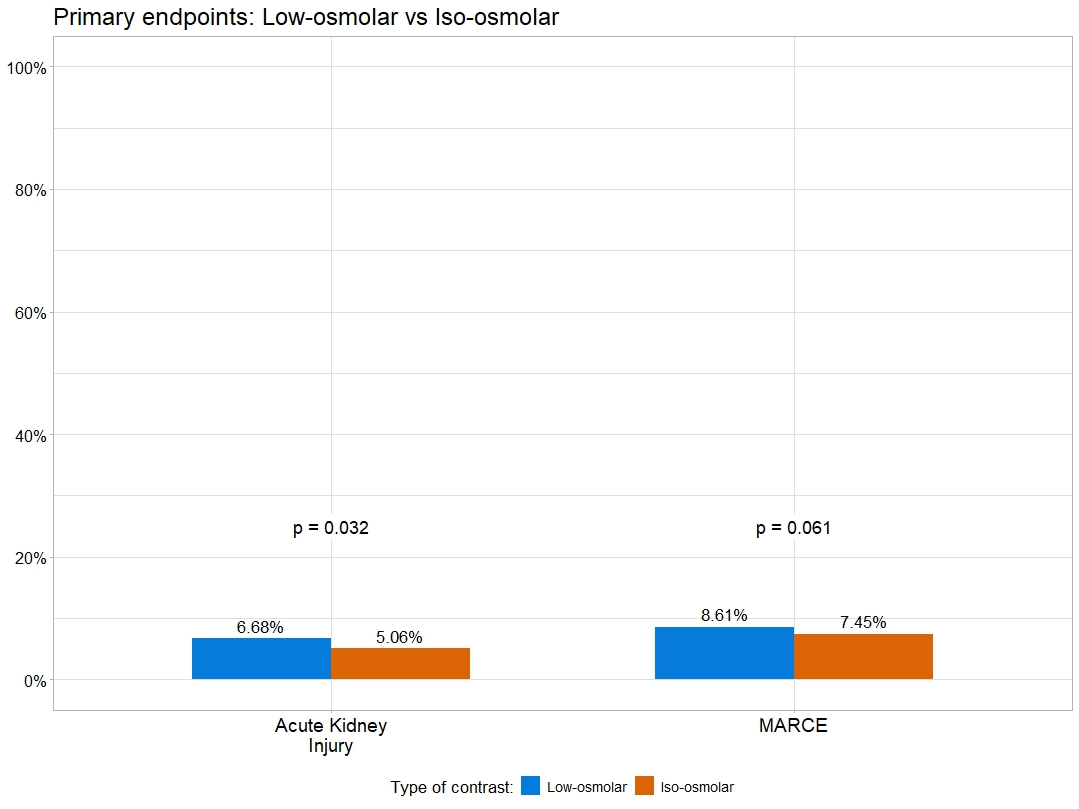
The incidence of AKI was 6.4%. After propensity matching, compared with patients who received low-osmolar contrast agents, iodixanol patients had a lower incidence of AKI (OR: 0.67; 95% CI, 0.47, 0.97; P = .032) and a trend for lower incidence of MARCE (OR: 0.75; 95% CI, 0.56, 1.00; P = .061) (Figure 2, Table 6). On multivariable analysis, iodixanol use was associated with a lower incidence of AKI (adjusted OR: 0.67; CI, 0.46, 0.96; P = .037) and MARCE (adjusted OR: 0.65; CI, 0.47, 0.91; P = .011). The incidence of new requirement for dialysis (0.0% vs 0.0%, P > .999), acute MI (0.7% vs 0.3%, P = .100), stent thrombosis (0.0% vs 0.1%, P > .999), stroke (0.3% vs 0.1%, P = .117), and death (0.3% vs 0.2%, P = .431) was similar between the 2 groups.

The incidence of AKI and MARCE for each contrast agent is shown in Figure 3. On multivariable analysis, the incidence of AKI with iodixanol was lower compared with iohexol but similar to iopromide and iopamidol (Figure 4). Similarly, iodixanol was associated with lower incidence of MARCE compared with iohexol, with a trend for lower incidence of MARCE compared with iopamidol and iopromide (Figure 5).



Discussion
The main findings of our study are that, in patients undergoing CTO PCI, iodixanol was used in patients with more comorbidities and higher angiographic complexity, was associated with lower technical and procedural success but similar MACE, and was associated with lower AKI after propensity matching and in multivariable analysis.
The incidence of AKI in our study was 6.1%, which is lower than reported in other studies.8,9 The exact mechanism by which contrast media are associated with AKI remains unclear; however, 3 major pathways have been implicated; the direct cytotoxic effect of contrast media on tubular cells, the alteration of renal hemodynamics, and the generation of reactive oxygen species.10 Iso-osmolar contrast media have been associated with lower incidence of AKI, possibly due to their more modest effect regarding osmotic diuresis, which could lead to reduced medullary stress and hypoxia or volume depletion compared with low-osmolar contrast media.11
CTO PCIs require longer procedure time and higher contrast volume compared with non-CTO PCIs and carry higher risk for AKI. Higher complexity cases require higher contrast volumes.12 CA-AKI and has been associated with worse follow-up outcomes after CTO PCI.13 In our study, despite higher complexity, iodixanol cases required longer procedure times but shorter fluoroscopy times. This could reflect the higher difficulty in crossing the CTO lesion. Use of femoral access was also higher in iodixanol cases, likely because of higher patient and angiographic complexity. CA-AKI has been associated with longer length of stay and over $10 000 in added costs.14 Minimizing the risk of CA-AKI in these procedures could at least in part mitigate the higher upfront cost of iodixanol.
The risk of AKI can be reduced by hydration15 and minimizing contrast volume.16,17 Our study suggests that use of iso-osmolar contrast media may be beneficial as well, even after adjusting for contrast volume. Prior studies have suggested lower incidence of AKI and MARCE with iso-osmolar contrast media in patients undergoing PCI as well as peripheral endovascular procedures,18,19 especially in patients at increased risk for AKI.20,21 Similar findings were observed in some but not all prior studies.22 Moreover, iodixanol was more likely to be administered to more complex patients and lesions. Lin et al showed that iodixanol was used in CTO PCI cases at higher risk for contrast-induced AKI and was associated with similar incidence of contrast-induced AKI.23 The potential benefits of iso-osmolar contrast media should be weighed against their higher cost compared with low-osmolar contrast media. Prior studies demonstrated that use of iso-osmolar contrast media may provide cost savings because of the high cost of AKI.24,25 This was also the case for patients at increased risk of CA-AKI.26
Limitations
Our study has limitations. There was no adjudication of events or angiographic variables by a core laboratory. The type of contrast agent used was at the discretion of the operator. As in any retrospective comparative efficacy study, there is a possibility of confounding, but results were similar in both propensity matching and multivariable analyses. There was no randomization and no systematic follow-up of renal function that may have led to underestimation of the incidence of AKI. Our registry gathers data from centers with expertise in CTO PCI, which may limit the generalizability of our results. Imputation of data can introduce systematic error.
Conclusions
The incidence of AKI in patients undergoing CTO PCI was 6.1%. Iodixanol was used in more complex patients and lesions and was associated with a lower incidence of AKI. The potential benefit of iodixanol must be confirmed in a randomized clinical trial comparing contrast agents.
Affiliations and Disclosures
Dimitrios Strepkos, MD1; Athanasios Rempakos, MD1; Michaella Alexandrou, MD1; Deniz Mutlu, MD1; Pedro E. P. Carvalho, MD1; Ozgur Selim Ser, MD1; Khaldoon Alaswad, MD2; Mir B. Basir, DO2; Dmitrii Khelimskii, MD3; Oleg Krestyaninov, MD3; Jaikirshan J. Khatri, MD4; Laura Young, MD4; Omer Goktekin5, MD; Paul Poommipanit, MD6; Farouc Jaffer, MD, PhD7; Sevket Gorgulu, MD8, Ahmed M. ElGuindy, MD9; Nidal Abi Rafeh, MD10; Olga Mastrodemos, BA1; Bavana V. Rangan, BDS, MPH1; Sandeep Jalli, DO1; Konstantinos Voudris1, MD, PhD; Yader Sandoval, MD1; M. Nicholas Burke, MD1; Emmanouil S. Brilakis, MD, PhD1
From the 1Minneapolis Heart Institute and Minneapolis Heart Institute Foundation, Abbott Northwestern Hospital, Minneapolis, Minnesota, 2Henry Ford Cardiovascular Division, Detroit, Michigan; 3Meshalkin Novosibirsk Research Institute, Novosibirsk, Russia; 4Cleveland Clinic, Cleveland, Ohio; 5Memorial Bahcelievler Hospital, Istanbul, Turkey; 6University Hospitals, Case Western Reserve University, Cleveland, Ohio;7Massachusetts General Hospital, Boston, Massachusetts;8Biruni University Medical School, Istanbul, Turkey; 9Aswan Heart Center, Aswan, Egypt; 10North Oaks Health System, Hammond, Louisiana.
Disclosures: Dr Jaffer conducts sponsored research for Canon, Siemens, Shockwave, Teleflex, Mercator, Boston Scientific, HeartFlow, and Neovasc; receives consultant/speakers fees from Boston Scientific, Siemens, Magenta Medical, Philips, Biotronik, Mercator, Terumo, Abiomed, Shockwave, DurVena, Intravascular Imaging, Inc., Medtronic, and FastWave; has equity interest in Intravascular Imaging, Inc., DurVena, and FastWave; has licensing arrangements with Massachusetts General Hospital; and has the right to receive royalites from Terumo, Canon, and SpectraWAVE. Dr Sandoval is a consultant for and on the advisory boards of Abbott and GE Healthcare; is a consultant for, on the advisory board of, and a speaker for Roche Diagnostics and Philips; is on the advisory board of Zoll; is a consultant for CathWorks; is a speaker for HeartFlow; is a speaker for and receives research grant from Cleerly; is an associate editor for JACC Advances; and holds patent 20210401347. Dr Brilakis receives consulting/speaker honoraria from Abbott Vascular, the American Heart Association (associate editor, Circulation), Biotronik, Boston Scientific, Cardiovascular Innovations Foundation (Board of Directors), Cordis, CSI, Elsevier, GE Healthcare, Haemonetics, IMDS, Medtronic, Teleflex, and Orbus Neich; receives research support from Boston Scientific and GE Healthcare; is the owner of Hippocrates LLC; and is a shareholder in LifeLens Technologies, Inc., MHI Ventures, Cleerly Health, Stallion Medical, and TrueVue, Inc.
Funding: The study was supported by a grant from GE HealthCare. The authors are thankful for the generous support of our anonymous donors, as well as Drs. Mary Ann and Donald A. Sens; Mr. Raymond Ames and Ms. Barbara Thorndike; the Frank J. and Eleanor A. Maslowski Charitable Trust; the Joseph F. and Mary M. Fleischhacker Family Foundation; Mrs. Diane and Dr. Cline Hickok; Mrs. Marilyn and Mr. William Ryerse; Mr. Greg and Mrs. Rhoda Olsen; the Wilma and Dale Johnson family; the Charlotte and Jerry Golinvaux Family Fund; the Roehl Family Foundation; and the Joseph Durda Foundation. Their support of the Minneapolis Heart Institute Foundation's Science Center for Coronary Artery Disease (CCAD) helped make this research project possible.
Address for correspondence: Emmanouil S. Brilakis, MD, PhD, Minneapolis Heart Institute, 920 E 28th Street #300, Minneapolis, MN 55407, USA. Email: esbrilakis@gmail.com
References
1. Isaac T, Gilani S, Kleiman NS. When prevention is truly better than cure: contrast-associated acute kidney injury in percutaneous coronary intervention. Methodist Debakey Cardiovasc J. 2022;18(4):73-85. doi:10.14797/mdcvj.1136
2. Jo SH. Iso-osmolar iodixanol is better than low-osmolar contrast for CIN prevention. And then? Korean Circ J. 2021;51(2):182-184. doi:10.4070/kcj.2021.0003
3. Morino Y, Abe M, Morimoto T, et al; J-CTO Registry Investigators. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J-CTO (multicenter CTO registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011;4(2):213-221. doi:10.1016/j.jcin.2010.09.024
4. Christopoulos G, Kandzari DE, Yeh RW, et al. Development and validation of a novel scoring system for predicting technical success of chronic total occlusion percutaneous coronary interventions: the PROGRESS CTO (Prospective Global Registry for the Study of Chronic Total Occlusion Intervention) score. JACC Cardiovasc Interv. 2016;9(1):1-9. doi:10.1016/j.jcin.2015.09.022
5. Simsek B, Kostantinis S, Karacsonyi J, et al. Predicting periprocedural complications in chronic total occlusion percutaneous coronary intervention: the PROGRESS-CTO complication scores. JACC Cardiovasc Interv. 2022;15(14):1413-1422. doi:10.1016/j.jcin.2022.06.007
6. McDonald JS, McDonald RJ, Comin J, et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119-128. doi:10.1148/radiol.12121460
7. Ybarra LF, Rinfret S, Brilakis ES, et al; Chronic Total Occlusion Academic Research Consortium. Definitions and clinical trial design principles for coronary artery chronic total occlusion therapies: CTO-ARC consensus recommendations. Circulation. 2021;143(5):479-500. doi:10.1161/CIRCULATIONAHA.120.046754
8. Tajti P, Ayoub M, Ahres A, et al. Procedural outcomes of chronic total occlusion percutaneous coronary interventions in patients with acute kidney injury. Cardiol J. 2024;31(1):84-94. doi:10.5603/CJ.a2022.0121
9. Azzalini L, Ojeda S, Demir OM, et al. Recanalization of chronic total occlusions in patients with vs without chronic kidney disease: the impact of contrast-induced acute kidney injury. Can J Cardiol. 2018;34(10):1275-1282. doi:10.1016/j.cjca.2018.07.012
10. Li Y, Wang J. Contrast-induced acute kidney injury: a review of definition, pathogenesis, risk factors, prevention and treatment. BMC Nephrol. 2024;25(1):140. doi:10.1186/s12882-024-03570-6
11. Aspelin P, Aubry P, Fransson SG, Strasser R, Willenbrock R, Berg KJ; Nephrotoxicity in High-Risk Patients Study of Iso-Osmolar and Low-Osmolar Non-Ionic Contrast Media Study Investigators. Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med. 2003;348(6):491-499. doi:10.1056/NEJMoa021833
12. Christakopoulos GE, Karmpaliotis D, Alaswad K, et al. Contrast utilization during chronic total occlusion percutaneous coronary intervention: insights from a contemporary multicenter registry. J Invasive Cardiol. 2016;28(7):288-294.
13. Sun G, Chen P, Wang K, et al. Contrast-induced nephropathy and long-term mortality after percutaneous coronary intervention in patients with acute myocardial infarction. Angiology. 2019;70(7):621-626. doi:10.1177/0003319718803677
14. Griffiths RI, Cavalcante R, McGovern AM, et al. Cost to Medicare of acute kidney injury in percutaneous coronary intervention. Am Heart J. 2023;262:20-28. doi:10.1016/j.ahj.2023.03.013
15. Moroni F, Baldetti L, Kabali C, et al. Tailored versus standard hydration to prevent acute kidney injury after percutaneous coronary intervention: network meta-analysis. J Am Heart Assoc. 2021;10(13):e021342. doi:10.1161/JAHA.121.021342
16. Soomro QH, Anand ST, Weisbord SD, et al; PRESERVE Trial Investigators; PRESERVE Trial Group. The Relationship between rate and volume of intravenous fluid administration and kidney outcomes after angiography. Clin J Am Soc Nephrol. 2022;17(10):1446-1456. doi:10.2215/CJN.02160222
17. Almendarez M, Gurm HS, Mariani J Jr, et al. Procedural strategies to reduce the incidence of contrast-induced acute kidney injury during percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;12(19):1877-1888. doi:10.1016/j.jcin.2019.04.055
18. Prasad A, Amin AP, Ryan MP, Gunnarsson C, Brilakis ES. Use of iso-osmolar contrast media during endovascular revascularization is associated with a lower incidence of major adverse renal, cardiac, or limb events. Catheter Cardiovasc Interv. 2022;99(4):1335-1342. doi:10.1002/ccd.30006
19. McCullough PA, David G, Todoran TM, Brilakis ES, Ryan MP, Gunnarsson C. Iso-osmolar contrast media and adverse renal and cardiac events after percutaneous cardiovascular intervention. J Comp Eff Res. 2018;7(4):331-341. doi:10.2217/cer-2017-0052
20. McCullough PA, Bertrand ME, Brinker JA, Stacul F. A meta-analysis of the renal safety of isosmolar iodixanol compared with low-osmolar contrast media. J Am Coll Cardiol. 2006;48(4):692-699. doi:10.1016/j.jacc.2006.02.073
21. Amin AP, Prasad A, Ryan MP, Gunnarsson C, Brilakis ES. Association of iso-osmolar vs low-osmolar contrast media with major adverse renal or cardiovascular events in patients at high risk for acute kidney injury undergoing endovascular abdominal aortic aneurysm repair. J Invasive Cardiol. 2021;33(8):E640-E646. doi:10.25270/jic/20.00670
22. Jovin IS, Warsavage TJ, Plomondon ME, et al. Iso-osmolar versus low-osmolar contrast media and outcomes after percutaneous coronary intervention: insights from the VA CART program. Catheter Cardiovasc Interv. 2022;100(1):85-93. doi:10.1002/ccd.30218
23. Lin YS, Fang HY, Hussein H, et al. Predictors of contrast-induced nephropathy in chronic total occlusion percutaneous coronary intervention. EuroIntervention. 2014;9(10):1173-1180. doi:10.4244/EIJV9I10A198
24. Hiremath S, Akbari A, Wells GA, Chow BJW. Are iso-osmolar, as compared to low-osmolar, contrast media cost-effective in patients undergoing cardiac catheterization? An economic analysis. Int Urol Nephrol. 2018;50(8):1477-1482. doi:10.1007/s11255-018-1874-1
25. Keuffel E, McCullough PA, Todoran TM, et al. The effect of major adverse renal cardiovascular event (MARCE) incidence, procedure volume, and unit cost on the hospital savings resulting from contrast media use in inpatient angioplasty. J Med Econ. 2018;21(4):356-364. doi:10.1080/13696998.2017.1415912
26. Aspelin P, Aubry P, Fransson SG, Strasser R, Willenbrock R, Lundkvist J. Cost-effectiveness of iodixanol in patients at high risk of contrast-induced nephropathy. Am Heart J. 2005;149(2):298-303. doi:10.1016/j.ahj.2004.07.020



















