Endothelialization of Amplatzer PFO Occluder Device 12 Months After Implantation: First-in-Human Angioscopic Assessment
Abstract
J INVASIVE CARDIOL 2022;34(2):E151.
Key words: transcatheter patent foramen ovale closure, angioscopy, endothelialization
Case Presentation
The current guidelines recommend at least 6 months of antithrombotic and antibiotic prophylaxis following atrial septal occluding device placement using the phrase “until endothelialization.” However, neo-endothelialization has not been assessed in vivo in humans. Considering the atrial septal defect occluding device, several autopsy cases and device extraction cases only demonstrated insufficient endothelialization beyond 6 months after implantation caused endocarditis and thrombosis. The effects of transcatheter patent foramen ovale (PFO) closure were established in 2017 and recently widely employed. The appropriate antithrombotic therapy after this novel procedure is entirely unclear.
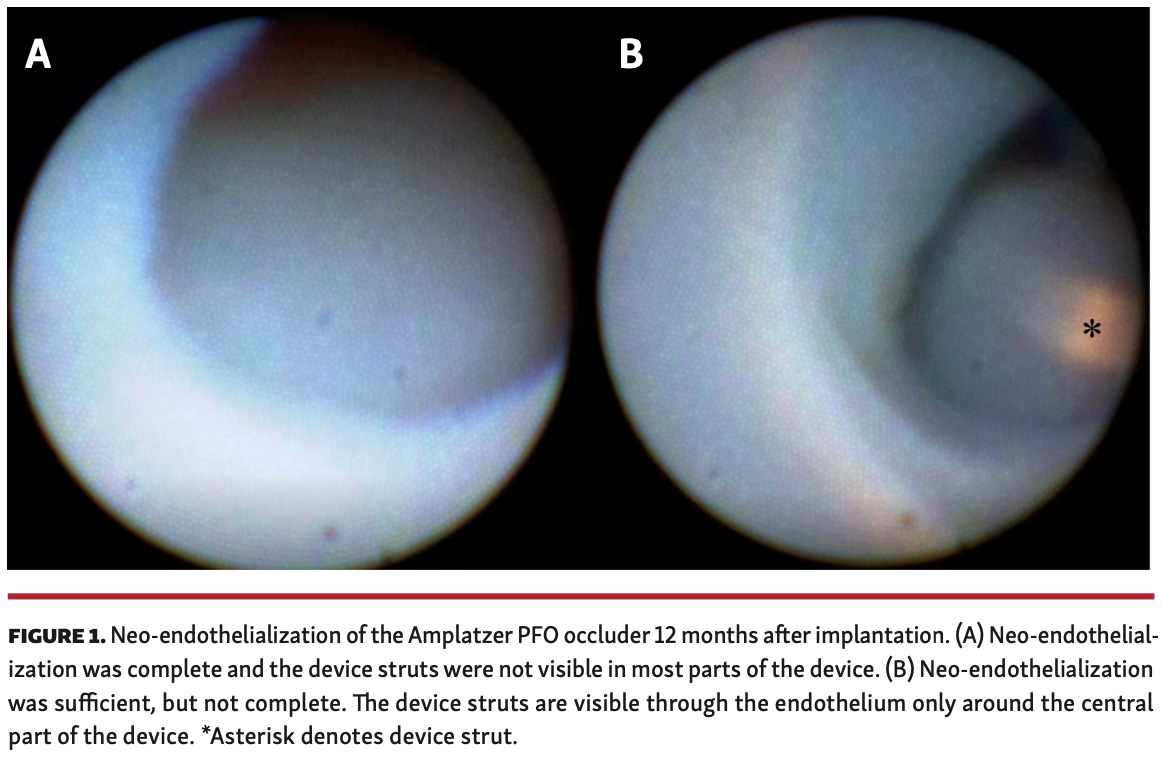
Accordingly, we have successfully developed a method for determining device endothelialization using angioscopy, which evaluated the endothelialization of a 25 mm Amplatzer PFO occluder device (Abbott) in a 40-year-old man 12 months after implantation. Angioscopy revealed that the neo-endothelialization was completed and the device struts were not visible in most parts of the device (Figure 1A; Video 1). The neo-endothelialization was sufficient, but not completed, and the device struts could be seen through the endothelium only around the central part (Figure 1B; Video 2). This is the first report evaluating the PFO occluder device in vivo. The neo-endothelialization was excellent 12 months after implantation. The antithrombotic therapy could be terminated unless residual shunt or the risks for stroke other than PFO existed.
Affiliations and Disclosures
From the Division of Cardiology, Department of Internal Medicine, St. Marianna University School of Medicine, Kanagawa, Japan.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript accepted September 8, 2021.
The authors report that patient consent was provided for publication of the images used herein.
Address for correspondence: Yasuhiro Tanabe, MD, PhD, Division of Cardiology, Department of Internal Medicine, St. Marianna University School of Medicine, 2-16-1, Sugao, Miyamae-ku, Kawasaki-City, Kanagawa 216-8511, Japan. Email: y-tanabe@muj.biglobe.ne.jp
Related Articles
- Propensity-Score Matched Comparison of the Cera PFO Occluder With the Amplatzer PFO Occluder for Percutaneous Closure of Patent Foramen Ovale Without Echocardiographic Guidance
- Endothelialization of an Amplatzer Septal Occluder Device 6 Months Post Implantation: Is This Enough Time? An In Vivo Angioscopic Assessment
- Delayed Iatrogenic Left Ventricular Apex Perforation Sealed With an Amplatzer Septal Occluder Device Under Transthoracic Echocardiography Guidance
















