Utilization of Collagen-Based Vascular Closure Devices in Patients With Severe Peripheral Artery Disease
Download a PDF of this article.
Abstract: Background. Collagen-based vascular closure devices (VCD) are commonly used after catheterization with femoral access. However, data about complication rates due to the utilization of VCDs in patients with known peripheral artery disease (PAD) of the lower limbs are inconsistent and patients with significant PAD are excluded in most VCD trials. In this study, we aimed to assess complication rates of collagen-based VCDs in patients with significant PAD. Methods. Patients with significant PAD treated with a VCD (Angio-Seal; St Jude Medical, Inc) after percutaneous therapeutic interventions of lower extremities were included in this study. Significant PAD was defined as Fontaine ≥2b. In-hospital complications (bleeding, spurious aneurysm, vessel occlusion, dissection, surgical repair, vasovagal reaction) were recorded. Results. A total of 121 patients (64.6 ± 11.3 years, 77% male) were included. PAD stage IIb was present in 99 patients (stage III in 8 patients, stage IV in 14 patients). A total of 112 treatments (93.3%) processed without complications (major complication rate, 1.7%; minor complication rate, 5.0%). There was a trend toward higher prevalence of complications with increasing size of closure device and with the stage of PAD; however, this trend was not statistically significant (P>.05 for all). Conclusion. We report moderate complication rates of collagen-based VCDs in patients with significant PAD. Our data suggest that Angio-Seal may be safe in patients with PAD after catheter intervention. Further randomized trials with larger sample size comparing VCD with standard manual compression in patients with significant PAD are required.
J INVASIVE CARDIOL 2013;25(1):19-22
Key words: vascular closure devices, peripheral arterial disease, femoral arterial access, AngioSeal
___________________________________________
Vascular procedures in interventional cardiology, neurology, and radiology are mostly performed via femoral arterial access. After procedures, manual compression of the femoral artery is a successful and still the most common method to achieve hemostasis.1-3
In order to reduce time to hemostasis and ambulation, confirming superior patient comfort, and to reduce complication rates after femoral access, vascular closure devices (VCDs) were developed in the 1990s.
Since that time, many studies and meta-analyses demonstrated that the use of VCDs — above all, the collagen-based Angio-Seal VCD (St Jude Medical, Inc) — in patients after cardiac catheterization with femoral access are safe and effective and that the risk of major complication is not increased compared to manual compression (MC).4-8 However, most of these trials excluded patients with significant peripheral arterial disease (PAD). Presence of significant PAD is suggested to be associated with an increased risk of complications;3 VCDs affect the inner arterial lumen, which may represent an extra source of complications in patients with PAD of the lower limbs.2,9 Therefore, patients with significant PAD are considered to be high risk patients for the use of VCD.3,10,11 Also, the suppliers of VCDs caution against the use of VCDs in patients with PAD.9
Compared to the use of VCDs after cardiac catheterization, data about safety and efficacy of VCDs in patients with significant PAD are rare. Only one systematic review and meta-analysis has been performed in interventional radiological procedures; it reported that the use of VCDs was associated with vascular complication rates similar to those following MC.12 In this work, the authors acknowledged the small number of prospective studies and the lack of inclusion of patients with PAD of the lower limbs, pointing toward the need for more prospective studies including patients with significant PAD undergoing interventions of the lower limbs.
Therefore, the aim of this prospective study was to assess the efficacy and safety of a collagen-based VCD (Angio-Seal) in patients with significant PAD undergoing an interventional procedure of the lower limbs and to determine the association of complications with stages of PAD.
Methods
Study design and patient selection. This study was a prospective, single-center, non-randomized analysis of consecutive patients with significant PAD undergoing an interventional therapeutic procedure of the lower limbs requiring femoral access. We included patients with significant PAD scheduled for intervention of the lower extremities and by which hemostasis was achieved using the collagen-based AngioSeal VCD.
Exclusion criteria were: treatment with Angio-Seal within the last 90 days; known allergy to bovine products; and a puncture adjacent to the femoral bifurcation. All other patients were treated with the collagen-based VCD and were included (n = 121). Patients with small artery size (<4 mm in diameter) were handled with care, but were not excluded (as per recommendation of the supplier).
Patient age, sex, and traditional risk factors (diabetes, hypertension, hyperlipidemia, and history of smoking) were recorded by systematic questioners. Before intervention, all patients were categorized according to Fontaine classification IIb-IV by physical examination and systematic questioners, with stage IIb or higher defined as significant PAD.
Technique. One physician (DL), who performed more than 100 Angio-Seal device applications before this study began, performed all interventions and Angio-Seal utilizations. Angio-Seal closure device deployment technique, as well as device description, are described elsewhere.13,14 In brief, the device consists of an absorbable intraluminal component (“anchor”) and a small collagen plug. The anchor is deployed intraluminally, the arterial wall and the arteriotomy site are “sandwiched” between the anchor and the collagen plug, and the collagen plug induces coagulation.
All interventional procedures in this trial were therapeutic; therefore, patients received 500 mg aspirin and 10000 U unfractionated heparin, as well as 75 mg clopidogrel if a stent placement was intended. The puncture site was disinfected using liquid betadine. After implementation of the local anesthetic, the arterial puncture was performed and a sheath was inserted. We used 6 Fr or 8 Fr sheaths for the procedures. Periprocedural antibiotics were not used. After the intervention and before removing the sheath, we performed an angiography of the puncture site. After exclusion of contraindications, sheaths were withdrawn immediately and the Angio-Seal closure device was used to achieve hemostasis. Additional compression bandages were not applied.
In-hospital follow-up. Following the therapeutic interventions, patients were requested to lie on their back for 6 hours. Patients were examined for access-site complications. The puncture site was inspected after 30 minutes, and at 2 and 8 hours. When no complications were noted, patients were discharged home with reference to report immediately if swelling, hematoma, or pain occurred.
Complications and device failure. The definition of major and minor complications has been previously defined and published.2,10,11,15-18 In brief, major complications were considered to be those requiring procedural or surgical intervention or bleeding requiring transfusion. Minor complications included any complication from the puncture site that was controlled via conservative management. Device failures were defined as bleeding persisting after deployment of the device, requiring subsequent MC.
Statistical analysis. Patient characteristics are presented as mean ± standard deviation (SD) for continuous variables and as n (%) for dichotomous traits. Statistical analysis was performed using Fisher’s exact test. A P-value of <.05 indicated statistical significance. SPSS version 12.0 was used for all computations.
Results
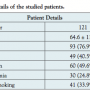 A total of 121 patients were included in this analysis (mean age, 64.6 ± 11.3 years; 77% male). Patient details were shown in Table 1. According to the Fontaine classification, 99 patients (81%) were classified as stage IIb, 8 patients (7%) as stage III and 14 patients (12%) as stage IV. All of the patients underwent a therapeutic procedure of the lower limbs (percutaneous transluminal angioplasty [PTA] and stenting in 82 patients; PTA only in 20 patients; other interventions including rotational ablation and a combination in 19 patients).
A total of 121 patients were included in this analysis (mean age, 64.6 ± 11.3 years; 77% male). Patient details were shown in Table 1. According to the Fontaine classification, 99 patients (81%) were classified as stage IIb, 8 patients (7%) as stage III and 14 patients (12%) as stage IV. All of the patients underwent a therapeutic procedure of the lower limbs (percutaneous transluminal angioplasty [PTA] and stenting in 82 patients; PTA only in 20 patients; other interventions including rotational ablation and a combination in 19 patients).
Six Fr sheaths were necessary in 99 patients and 8 Fr sheaths were used in 22 patients.
In 1 patient, the application of the VCD failed and hemostasis was achieved by MC. Overall, the application of the VCD was successful in 99.2%. Regarding the complications, we analyzed 120 patients in which the application of the VCD was successful.
After utilization of Angio-Seal, 2 patients developed major complications. In 1 patient with stage IIb PAD, PTA and stenting of the common iliac artery using a 6 Fr sheath was performed. The VCD was inserted after angiography of the puncture site. The follow-up exams were inconspicuous. The next day, the patient complained about pain in the lower leg. Magnetic resonance imaging and angiography showed a stenosis of the puncture site. A short dissection was responsible for the stenosis, identified in subsequently performed surgery. The anchor of the VCD was adherent to the posterior vascular wall, which caused the dissection. One week after surgical treatment, the patient was discharged free of symptoms. A second patient had a total occlusion of the vessel after Angio-Seal utilization. Bilateral PTA and stenting were performed using an 8 Fr sheath. In the first control, there was no pulse at the puncture site of the lower limbs. Computed tomography angiography suggested a total occlusion of the femoral artery. This was caused by the anchor and collagen plug located intravasally, triggering a thrombogenic occlusion of the vessel. The patient was discharged 8 days after surgery.
Minor complications occurred in 6 patients. Three developed small hematoma, without a decrease of hemoglobin levels. Another patient had a spurious aneurysm, which was treated with MC and compression bandages for 24 hours with no spurious aneurysm detectable on duplex sonography the next day. Two patients had a vasovagal reaction during application of the VCD. These patients were treated with an infusion and atropine.
Overall, 2 patients had major complications (1.7%; 95% confidential interval [CI] 0%-4.0%) and 6 patients had minor complications (5.0%; 95% CI, 1.1%-8.9%).
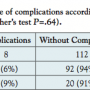
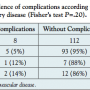 The association of PAD stage with complication rates is shown in Table 2. There was a trend toward higher frequency of complications with higher stages of PAD without reaching statistical significance (PAD stage IIb, 5%; stage III, 12%; stage IV, 14%; P=.20). Likewise, complication rates were higher using 8 Fr sheaths compared to 6 Fr sheaths, again without reaching statistical significance (6 Fr, 6.1%; 8 Fr, 9.1%; P=.63) (Table 3).
The association of PAD stage with complication rates is shown in Table 2. There was a trend toward higher frequency of complications with higher stages of PAD without reaching statistical significance (PAD stage IIb, 5%; stage III, 12%; stage IV, 14%; P=.20). Likewise, complication rates were higher using 8 Fr sheaths compared to 6 Fr sheaths, again without reaching statistical significance (6 Fr, 6.1%; 8 Fr, 9.1%; P=.63) (Table 3).
In 11 patients, a bilateral intervention of the lower limbs was performed. Of these 11 patients, 3 developed complications, resulting in a higher complication rate after bilateral compared to unilateral interventions (27.3% vs 4.6%; P=.03).
Discussion
 In this prospective study, we determined complication rates due to the utilization of the Angio-Seal VCD in patients with known PAD, undergoing an interventional procedure of the lower limbs. We report moderate complication rates of collagen-based VCDs in patients with significant PAD (major complications in 1.7%, minor complications in 5.0%). There was a trend toward higher prevalence of complications with increasing size of closure device and with the stage of PAD; however, this trend did not reach statistical significance. Patients with bilateral interventions had higher complication rates compared to unilateral procedures.
In this prospective study, we determined complication rates due to the utilization of the Angio-Seal VCD in patients with known PAD, undergoing an interventional procedure of the lower limbs. We report moderate complication rates of collagen-based VCDs in patients with significant PAD (major complications in 1.7%, minor complications in 5.0%). There was a trend toward higher prevalence of complications with increasing size of closure device and with the stage of PAD; however, this trend did not reach statistical significance. Patients with bilateral interventions had higher complication rates compared to unilateral procedures.
After cardiac catheterization with femoral access, MC after removal of the sheath is still the gold standard,1-3 but the use of VCDs after cardiac catheterization since their introduction in the 1990s is widespread and further increasing.4-8 With growing experience and further development of VCDs, many studies documented that the use of VCDs is safe and effective after cardiac catheterization with risk of major complications comparable to MC. However, most literature on VCD safety is based on studies excluding patients with significant PAD.19-23 It was suggested that the presence of PAD is associated with higher frequency of complications after the utilization of a VCD.3 Patients with a significant PAD were considered to be high risk for the use of VCDs and even the suppliers of VCDs caution against the use of VCDs in patients with PAD.9
Compared to the use of VCDs after cardiac catheterization, data about the safety and efficacy due the utilization of VCDs in patients with a significant PAD are rare. Das et al published a systematic review and meta analysis in 2010 including all reported interventional radiological procedures examining complication rates when comparing the use of VCDs with MC.12 In this meta-analysis, non-comparative and comparative studies (VCD vs MC) were analyzed. A total of 1528 patients in whom hemostasis was achieved by a collagen-based closure device were included from non-comparative studies. Of these 1528, only 234 patients were rendered from prospective studies containing an intervention of the lower limbs. From the comparative studies (VCD vs MC), 267 patients with significant PAD receiving a VCD were analyzed from prospective studies. Moreover, all peripheral interventions were subsumed — interventions of the lower limbs were not considered separately. This meta analysis demonstrated the need for more prospective studies including patients with significant PAD of the lower limbs. With regard to the complication rates, there were no significant differences comparing VCD with MC.
In our prospective study, we report a moderate major complication rate (1.7%) after the utilization of a collagen-based VCD, which is comparable to the major complication rates in the current literature, ranging from 0% to 3.6% in subjects without severe PAD.24-30 In another trial, Silber analyzed the safety and success rate of VCDs in 6007 patients after cardiac catheterization.31 After utilization of the collagen-based VCD, the major complication rate was 1.8% and the minor complication rate was 6.7%. Overall, we report comparable complication rates using collagen-based VCDs in patients with PAD when compared to VCDs in patients without known PAD.
We observed higher complication rates for bilateral intervention compared to unilateral intervention. This effect might be caused by the longer sheath in-dwelling time after bilateral intervention. Previous studies demonstrated an association of sheath in-dwelling time with vascular complications.10,32-34
Moreover, in our study, there was a trend toward increased complication rates with higher stages of PAD. However, these findings were limited by the small number of patients with a stage III and IV PAD, with most of the patients considered to be stage IIb. Studies with larger numbers of patients with higher stages of PAD are needed to confirm our results. A further limitation of our study is the absence of a control group and the small sample size.
Conclusion
We report moderate complication rates of collagen-based VCDs in patients with significant PAD. Our data suggest that Angio-Seal may be safe in patients with PAD after catheter intervention. Further randomized trials with larger sample sizes comparing VCD with standard MC in patients with significant PAD are required.
References
- Tavris DR, Gallauresi BA, Lin B, et al. Risk of local adverse events following cardiac catheterization by hemostasis device use and gender. J Invasive Cardiol. 2004;16(9):459-464.
- G. Ansel, Yakubov S, Neilsen C, et al. Safety and efficacy of staple-mediated femoral arteriotomy closure: results from a randomized multicenter study. Catheter Cardiovasc Interv. 2006;67(4):546-553.
- Dauerman HL, Applegate RJ, Cohen DJ. Vascular closure devices: the second decade. J Am Coll Cardiol. 2007;50(17):1617-1626.
- Madigan JB, Ratnam LA, Belli AM. Arterial closure devices. A review. J Cardiovasc Surg. 2007;48(5):607-624.
- Koreny M, Riedmüller E, Nikfardjam M, et al. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization: systematic review and meta-analysis. JAMA. 2004;291(3):350-357.
- Nikolsky E, Mehran R, Halkin A, et al. Vascular complications associated with arteriotomy closure devices in patients undergoing percutaneous coronary procedures: a meta-analysis. J Am Coll Cardiol. 2004;44(6):1200-1209.
- Vaitkus PT. A meta-analysis of percutaneous vascular closure devices after diagnostic catheterization and percutaneous coronary intervention. J Invasive Cardiol. 2004;16(5):243-246.
- Behan MW, Large JK, Patel NR, et al. A randomised controlled trial comparing the routine use of an Angio-Seal STS device strategy with conventional femoral haemostasis methods in a district general hospital. Int J Clin Pract 2007;61(3):367-372.
- Angio-Seal Vascular Closure Device (2010). Instructions for use. www.sjmproffesional.com
- Piper WD, Malenka DJ, Ryan TJ Jr, et al. Predicting vascular complications in percutaneous coronary interventions. Am Heart J. 2003;145(6):1022-1029.
- Applegate RJ, Sacrinty M, Kutcher MA, et al. Vascular complications with newer generations of angioseal vascular closure devices. J Interv Cardiol. 2006;19(1):67-74.
- Das R, Ahmed K, Athanasiou T, et al. Arterial closure devices versus manual compression for femoral haemostasis in interventional radiological procedures: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2011;34(4):723-738.
- Henry M, Amor M, Allaoui M, Tricoche O. A new access site management tool: the Angio-Seal hemostatic puncture closure device. J Endovasc Surg. 1995;2(3):289-296.
- Beyer-Enke SA, Söldner J, Zeitler E, et al. Immediate sealing of arterial puncture site following femoropopliteal angioplasty: a prospective randomized trial. Cardiovasc Intervent Radiol. 1996;19(6):406-410.
- Geyik S, Yavuz K, Akgoz A, et al. The safety and efficacy of the Angio-Seal closure device in diagnostic and interventional neuroangiography setting: a single-center experience with 1,443 closures. Neuroradiology. 2007;49(9):739-746.
- Wagner SC, Gonsalves CF, Eschelman DJ, et al. Complications of a percutaneous suture-mediated closure device versus manual compression for arteriotomy closure. A case controlled study. J Vasc Interv Radiol. 2003;14(6):735-741.
- Sacks D, Marinelli DL, Martin LG, Spies JB. Reporting standard for clinical evaluation of new peripheral arterial revascularization devices. J Vasc Intervent Radiol. 1997;8(1 Pt 1):137-149.
- Leoni CJ, Potter JE, Rosen MP, et al. Classifying complications of interventional procedures: a survey of practicing radiologists. J Vasc Intervent Radiol. 2001;12(1):55-59.
- Boccalandro F, Assali A, Fujise K, Smalling RW, Sdringola S. Vascular access site complications with the use of closure devices in patients treated with platelet glycoprotein IIb/IIIa inhibitors during rescue angioplasty. Catheter Cardiovasc Interv. 2004;63(3):284-289.
- Chevalier B, Lancelin B, Koning R, et al. Effect of closure device on complication rates in high-local-risk patients: results of a randomized multicenter trial. Catheter Cardiovasc Interv. 2003;58(3):285-291.
- Eggebrecht H, von Birgelen C, Naber C, et al. Impact of gender on femoral access complications secondary to application of a collagen-based vascular closure device. J Invasive Cardiol. 2004;16(5):247-250.
- Reddy BK, Brewster PS, Walsh T, Burket MW, Thomas WJ, Cooper CJ. Randomized comparison of rapid ambulation using radial, 4 French femoral access, or femoral access with AngioSeal closure. Catheter Cardiovasc Interv. 2004;62(2):143-149.
- Silber S, Dörr R, Mühling H, et al. Sheath pulling immediately after PTCA: comparison of two different deployment techniques for the hemostatic puncture closure device, A prospective, randomized study. Cathet Cardiovasc Diagn. 1997;41(4):378-383.
- Ratnam LA, Raja J, Munneke GJ, et al. Prospective nonrandomized trial of manual compression and Angio-Seal and Starclose arterial closure devices in common femoral punctures. Cardiovasc Intervent Radiol. 2007;30(2):182-188.
- Park Y, Roh HG, Choo SW, et al. Prospective comparison of collagen plug (Angio-Seal) and suture-mediated (the Closer S) closure devices at femoral access sites. Korean J Radiol. 2005;6(4):248-255.
- Eidt JF, Habibipour S, Saucedo JF, et al. Surgical complications from hemostatic puncture closure devices. Am J Surg. 1999;178(6):511-516.
- Aksoy M, Becquemin JP, Desgranges P, et al. The safety and efficacy of Angio-Seal in therapeutic endovascular interventions. Eur J Vasc Endovasc Surg. 2006;32(1):90-93.
- Shammas NW, Rajendran VR, Alldredge SG, et al. Randomized comparison of Vasoseal and Angio-Seal closure devices in patients undergoing coronary angiography and angioplasty. Cathet Cardiovasc Interv. 2002;55(4):421-425.
- Bown MJ, Blanshard KS, Cutress ML, et al. Off-license use of Angio-Seal arterial puncture closure device. Eur J Vasc Endovasc. 2002;24(4):372-373.
- Shrake KL. Comparison of major complication rates associated with four methods of arterial closure. Am J Cardiol. 2000;85(8):1024-1025.
- Silber S. Hemostasis success rates and local complications with collagen after femoral access for cardiac catheterization: analysis of 6007 published patients. Am Heart J. 1998;135(1):152-156.
- Omoigui NA, Califf RM, Pieper K, et al. Peripheral vascular complications in the Coronary Angioplasty Versus Excisional Atherectomy Trial (CAVEAT-I). J Am Coll Cardiol. 1995;26(4):922-930.
- Popma JJ, Satler LF, Pichard AD, et al. Vascular complications after balloon and new device angioplasty. Circulation. 1993;88(4 Pt 1):1569-1578.
- Moscucci M, Mansour KA, Kent KC, et al. Peripheral vascular complications of directional coronary atherectomy and stenting: predictors, management, and outcome. Am J Cardiol. 1994;74(5):448-453.
_______________________________________________________
From the 1West-German Heart Center Essen, Department of Cardiology, University of Duisburg-Essen, Germany, ²Institute for Medical Informatics, Biometry and Epidemiology, University of Duisburg-Essen, Germany, and ³Department of Radiology, Augusta-Hospital Bochum, Germany.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Kahlert is a clinical proctor for Edwards Lifesciences and discloses travel expenses paid by Edwards Lifesciences, Medtronic, Inc, and Abbott Vascular.
Manuscript submitted April 23, 2012, provisional acceptance given May 4, 2012, final version accepted May 30, 2012.
Address for correspondence: Kaffer Kara, MD, West German Heart Center, Department of Cardiology, Hufelandstr. 55, 45147 Essen, Germany. Email: Kaffer.Kara@uk-essen.de


















