Use of MitraClip to Target Obstructive SAM in Severe Diffuse-Type Hypertrophic Cardiomyopathy: Case Report and Review of Literature
Abstract: Hypertrophic cardiomyopathy (HCM) is frequently associated with abnormalities of the mitral valve; these commonly include systolic anterior motion (SAM) of anterior mitral leaflets that contribute to dynamic left ventricular outflow tract (LVOT) obstruction and secondary mitral regurgitation (MR). In patients with severe HCM, LVOT obstruction due to SAM, and debilitating symptoms refractory to medical therapy, the current standard of care involves a surgical approach. This involves targeting the ventricular septum through resection or ablation, combined at times with mitral valve replacement or plication of the valve leaflet to relieve LVOT obstruction. In patients with symptoms refractory to medical management who are at prohibitive surgical risk, additional options for less-invasive approaches for the management of HCM are needed. We describe here the successful non-surgical catheter-based management of a 72-year-old woman at high surgical risk, debilitating symptoms refractory to maximal medical management, and severe, diffuse-type HCM. Edge-to-edge repair with MitraClip (Abbott Vascular) was used to target SAM causing dynamic LVOT obstruction, with resulting significant reduction in LVOT gradient and dramatic clinical improvement. Her postprocedure outcomes to 2 years are reported herein. Additionally, we review the current management strategies for HCM management, and include a discussion of minimally invasive options.
J INVASIVE CARDIOL 2020;32(9):E228-E232. doi:10.25270/jic/20.00157
Key words: edge-to-edge repair, HCM, MitraClip, systolic anterior motion
A transcatheter approach to mitral valve repair with the MitraClip device (Abbott Vascular) was recently approved by the United States Food and Drug Administration for patients with hemodynamically significant mitral regurgitation (MR) who are considered high risk for conventional surgical valve repair.1-3 This involves catheter-based edge-to-edge repair that affixes the anterior and posterior leaflets to reduce MR. Because this technique tethers the anterior leaflet to the posterior leaflet, it can reduce or completely prevent systolic anterior motion of the mitral leaflet.1-3 In proof of concept and early studies, MitraClip was employed in hypertrophic cardiomyopathy (HCM) patients with obstructive systolic anterior motion (SAM) to reduce left ventricular outflow tract (LVOT) gradient and to provide symptomatic relief, with promising early results of efficacy and feasibility, and increased quality of life.1-3 To date, no formal clinical trials have studied the use of MitraClip to target the obstructive mitral leaflet anomalies of HCM, and more information is needed regarding intermediate and long-term outcomes of this approach for HCM. Furthermore, a comparison of MitraClip’s efficacy in comparison with alcohol septal ablation (ASA), another minimally invasive approach for HCM, has not yet been undertaken, although, at least in theory, an edge-to-edge repair approach for relieving LVOT obstruction would avoid the potential for secondary conduction anomalies commonly seen after ASA.3,4 Additionally, use of MitraClip in place of ASA would alleviate the use of chemical agents for directed myocardial necrosis for left ventricular remodeling to occur.3,4
We discuss here the use of MitraClip to reduce mitral SAM for significant improvement of symptoms associated with LV obstruction in a patient at prohibitively high surgical risk, with outcomes reported to 2 years post procedure. We also review the current literature on this technique for the management of symptomatic HCM with SAM.
Case Report
A 72-year-old Caucasian woman (body mass index, 34.5 kg/m2; body surface area, 2.13 m2) with past medical history notable for moderate-severe MR (effective regurgitant orifice area, 0.35 cm2; regurgitant fraction, 41%; regurgitant volume, 45.6 mL), atrial fibrillation, bradycardia, and HCM with severe LVOT gradient at rest was referred to our tertiary-care center for consideration of mitral valve replacement. She admitted chronic but progressive symptoms of dyspnea, at first exertional but at presentation progressive and significant at rest. She was essentially wheelchair bound, given her profound dyspnea with any activity. A single-chamber pacemaker had been placed in the weeks prior for management of bradycardia; symptoms were unchanged despite escalating doses of verapamil after pacer placement. Her work-up prior to presentation included two-dimensional echocardiogram that showed normal LV systolic function with ejection fraction (EF) of 61%, mitral annular calcification (MAC) with a calcified posterior leaflet, severe MR with prominent SAM, and LVOT with peak gradient of 94 mm Hg during Valsalva maneuver.
Subsequent work-up included transesophageal echocardiogram (TEE), which showed normal EF, but revealed SAM with dynamic LVOT obstruction and moderate-severe MR (Figure 1A). Subsequent catheterization revealed patent coronary arteries. A dobutamine stress echocardiography was performed that showed elevated baseline LVOT gradients (72 mm Hg) on provocation with 10 µg dobutamine. A peak atrioventricular gradient of 171 mm Hg was noted with maximum velocity of 654 m/s (Figure 1B) with dynamic LVOT obstruction and SAM (Figures 1C and 1D). At the time of provocation, the patient reported her typical clinical symptoms and was highly symptomatic.
The patient was seen in a multidisciplinary valve clinic to consider management options. A low-profile mitral valve replacement was discussed to address the patient’s primary mitral valve disease; however, her surgical risk was felt to be high, with additional concern of frailty and other comorbidities (STS score was 7.2% and patient was a 3 out of 4 in frailty criteria [gait, grip, walk]). Given the normal size of the left atrium (22 cm2), prominent SAM, and dynamic LVOT obstruction, the consensus was that the patient’s anatomy was amenable to an edge-to-edge repair approach with MitraClip, with a back-up plan of ASA if MitraClip failed to yield expected LVOT gradient reduction.
Procedural Details
Transcatheter plication of the mitral valve with MitraClip implantation was performed as previously described.1,2 Briefly, an 8 Fr sheath was placed in the right femoral and access obtained with a trans-septal approach. Preintervention LVOT gradients were measured with end-hole catheters in the LV and aorta, using simultaneous hemodynamic monitoring. The MitraClip delivery catheter was brought across the septum to the left atrium with a 24 Fr Evalve sheath (Abbott Vascular), and both TEE and fluoroscopic guidance were used to carefully position the delivery catheter above the mitral valve. Three-dimensional imaging was then used to position and center the arms of the clip, which were then extended and advanced into the left atrium. Once position of the arms was confirmed a final time, the leaflets of the mitral valve were grasped at A2/P2 and preliminary assessment of hemodynamics with the device still attached was done. We were careful to assess reduction in SAM. Lastly, the gripper arms were released and the MitraClip device was deployed. LV and aortic pressures were then measured. Postinterventional hemodynamics were measured as 131/70 mm Hg in the aorta and 128/9 mm Hg in the LV upon subsequent provocation with 10 µg dobutamine; postdobutamine pressures were recorded as 136/73 mm Hg in the aorta and 134/9 mm Hg in the LV.
Follow-up TEE immediately post procedure showed only mild MR with significant improvement of SAM, both at baseline and with dobutamine provocation (Figure 2). On postoperative day 1, repeat transthoracic echocardiogram (TTE) showed EF of 75%, mitral valve peak velocity of 2 m/s, mean gradient of 5 mm Hg, and LVOT resting gradient of 27 mm Hg (down from 72 mm Hg); importantly, this gradient no longer increased with Valsalva maneuver. There was no residual evidence of SAM. Her postoperative recovery was uneventful and she was discharged on postoperative day 1 in stable condition. At 30-day follow-up exam, the patient had near-complete resolution of debilitating dyspnea and no longer required use of a wheelchair or home oxygen. Repeat TTE showed an unchanged EF of 75%, no evidence of SAM, mild MR, and a mean MV gradient of 5 mm Hg. LVOT gradient at rest was again noted at 27 mm Hg, and was unchanged with Valsalva maneuver.
Discussion
It has been recently estimated that approximately one-half of all HCM cases involve the enlargement and extension of mitral valve leaflets in the direction of the LVOT, creating obstruction and leading to valve regurgitation and progressive symptoms of heart failure. Currently, few therapeutic options exist for patients with hemodynamically significant HCM whose symptoms persist despite maximal medical treatment.
Septal reduction therapies for medically refractory HCM surgical myectomy. In patients under the age of 21 years, or those whose medical complexities do not preclude a surgical approach to management, septal myectomy (the “Morrow” procedure) remains the current standard of care for definite management and long-term symptom relief.5,6 In this procedure, a median sternotomy and cardiopulmonary bypass are employed to access the aorta and LV, with a transverse aortotomy made for the resection of the hypertrophied ventricular septum. In the subset of patients with coexisting degenerative mitral valve disease and hemodynamically significant HCM, concurrent valve replacement or mitral leaflet plication for subaortic obstruction from an elongated anterior mitral leaflet should be performed.5 Septal myectomies are performed in a small number of specialty centers, with survival rates that vary by center volume and experience; however, one recent study from a large HCM specialty center demonstrated survival rates as high as 98% at 1 year and 91% at 10 years post procedure.5
Alcohol septal ablation. For patients at prohibitively high surgical risk and in whom intrinsic mitral valve disease has been ruled out, ASA is one potential option for septal reduction in symptomatic HCM. In this minimally invasive approach, 1-4 mL of 96% ethanol is slowly infused into the first septal perforating branch of the left anterior descending coronary artery to induce controlled myocardial scarring of the basal septum with subsequent LV remodeling.4,6 Early and mid-term follow-up studies have demonstrated resulting thinning of the basal septum, significant reduction of LVOT gradients, and patient reports of improved symptoms, with an efficacy comparable with surgical myectomy.4 However, the incidences of postprocedure complete heart block, ventricular arrhythmia, and need for permanent pacemaker are increased compared with myectomy.4,6 Furthermore, ASA relies exclusively on favorable coronary anatomy, and significant variations can be seen in the myocardial territory supplied by the septal perforator, which may create unintended necrosis of the right ventricular septum or LV apex in addition to the intended basal septum target.4,6 Additionally, few studies are available to compare long-term outcomes of ASA compared with septal myectomy, and the potential need for surgical reintervention after ASA, especially in younger patients, cannot be overlooked.
Percutaneous edge-to-edge repair with leaflet plication. Early successes with the use of the MitraClip device to attenuate the late systolic motion of the anterior leaflet with a catheter-based edge-to-edge repair approach have been demonstrated beautifully by Sorajja and others.1-3,7 For the subset of patients who are poor surgical candidates with symptomatic HCM and intrinsic mitral valve disease amenable to repair, the use of the MitraClip transcatheter technique holds promise.1-3 With an entirely percutaneous approach, the anterior leaflet can be secured to the posterior leaflet, while allowing for an in vivo response by the operator in real time by echocardiography. The ability to release and reposition the clip also offers a low-risk approach to gradient reduction and symptomatic relief.1,2,7 From a technical perspective, implantation of the clips during edge-to-edge repair is still performed in the same manner, with orientation of the clip arms orthogonal to the commissural plane of the valve, just as described for patients with conventional functional or degenerative MR.1,7
Early reports1-3 have demonstrated that the plication of aberrant valve leaflets can successfully relieve LVOT obstruction and reduce valvular gradients, with corresponding improved clinical state (ie, improved New York Heart Association symptom class), and without the disruption of the cardiac conduction system that follows ASA.1-4 Important considerations during preprocedure evaluation for MitraClip in any HCM should include left atrial size, as many HCM patients have a smaller left atrium, which can pose a navigational challenge for the MitraClip operator.4 In the instance of the current patient, her left atrium was within a normal size range, so this was fortunately not an issue.
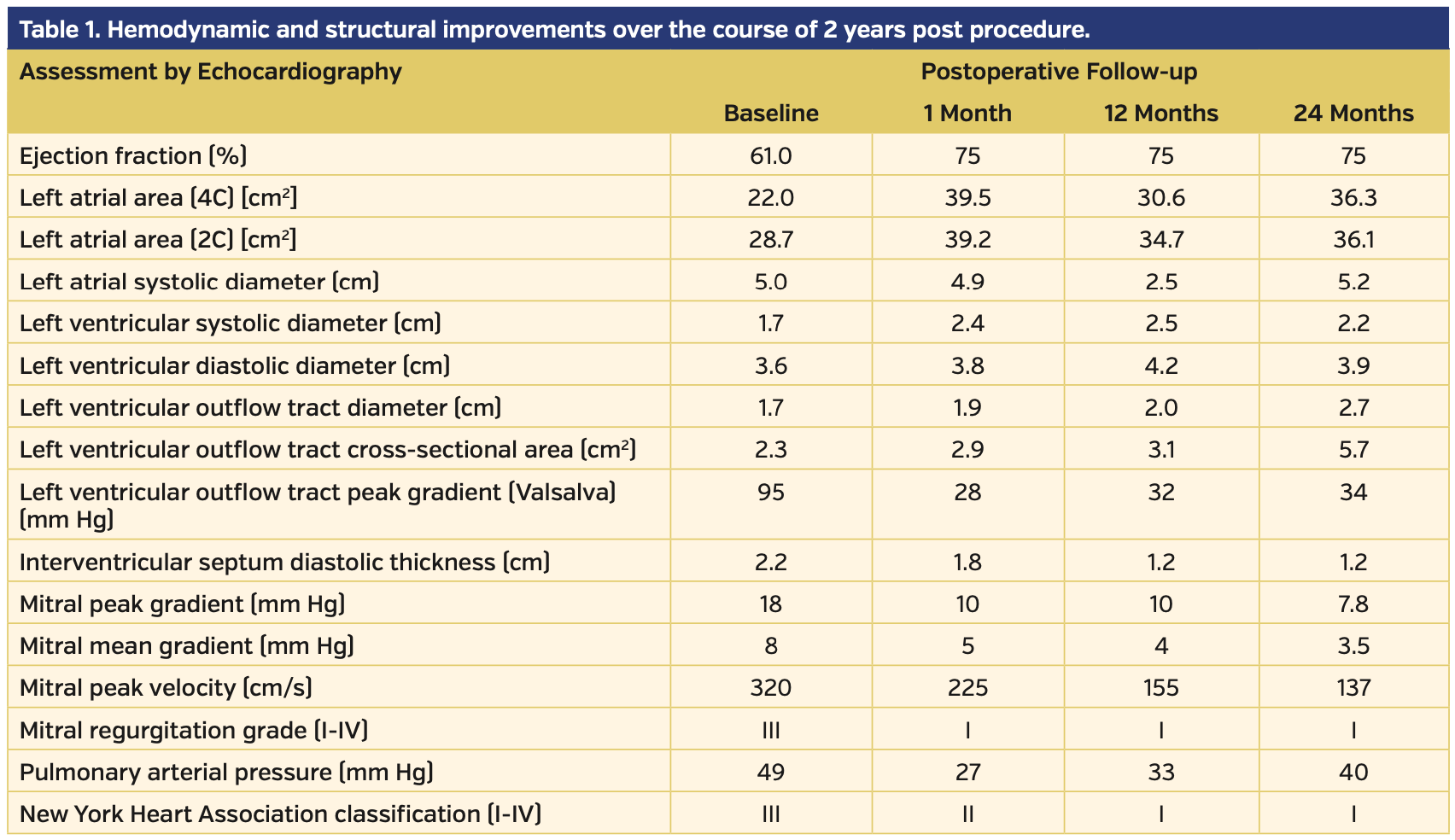
Given that long-term results using MitraClip for HCM and LVOT obstruction are not yet known, even if an edge-to-edge repair strategy proved ultimately ineffective for the relief of symptoms, most surgical options are still available for patients with refractory symptoms after MitraClip placement. In our patient, hemodynamic and structural improvement were noted over the course of 2 years, in addition to symptomatic improvement (Table 1).
To date, there are no other intermediate-term data available on the use of MitraClip for HCM. At least one early study1 has demonstrated persistently elevated LVOT gradients post MitraClip in 3 patients, which were not seen when invasive measurements were repeated.1 The authors postulated this might be secondary to a Doppler artifact (ie, pressure recovery), seen in much lower Doppler-derived LVOT gradients noted on invasive pressure assessments in at least 1 patient.5 One possible explanation for elevated LVOT gradients after MitraClip might be a persistent intracavitary gradient or “contamination” of the Doppler signal with residual MR.5 More research is needed in this area to better understand this finding. There is no comparative assessment of MitraClip and ASA for determination of the most effective minimally invasive strategy for treating the underlying causes of HCM. Given over 20 years of patient experience with ASA, it may be reasonable to consider this approach first in patients with HCM and with absent or mild intrinsic mitral valve disease. More data are needed to determine the long-term efficacy of MitraClip for HCM and whether subsequent interventions with repeat clip, ASA, or myectomy are needed.
We have added to the small body of the existing literature the case of a highly symptomatic patent with HCM and SAM successfully treated with transcatheter edge-to-edge repair with MitraClip, with substantial reduction in gradients and dramatic clinical benefit, consistently sustained over 2 years post procedure.
Study limitations. There are a number of limitations to our study, including the small sample size and lack of randomization. Furthermore, given the patient’s anatomical suitability for MitraClip, her accompanying significant frailty, and her persistent severity of symptoms, ASA was not employed first in this patient with high surgical risk, as it might otherwise have been in a more robust patient with accompanying preventative factors for surgery. It is difficult to postulate how this patient’s result might otherwise compare with a patient who underwent ASA, and as noted earlier, there are no current clinical trials in place to compare the efficacy, feasibility, and potential of long-term symptom relief with MitraClip vs ASA. Additionally, in patients who may be able to tolerate ASA, the combination of ASA with MitraClip is one future possibility for an effective treatment option for LVOT obstruction in HCM patients, but to our knowledge, this has not been shown in the literature to date.
Conclusion
Use of the MitraClip system to reduce mitral SAM underlying LVOT obstruction in symptomatic HCM patients may offer an additional option for management of severe symptoms in patients who are poor surgical candidates and lack other options. Larger clinical investigations of this technique using edge-to-edge repair to manage symptomatic HCM with SAM as an alternative to surgery are needed to better assess feasibility and safety.
From the Sentara Heart Hospital, Norfolk, Virginia.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript accepted April 9, 2020.
The authors report that patient consent was provided for publication of the images used herein.
Address for correspondence: Paul Mahoney, MD, Sentara Heart Valve and Structural Disease Center, Sentara Heart Hospital, 600 Gresham Drive, Norfolk, VA, 23507. Email: Paul.mahoney.md@gmail.com
- Sorajja P, Pedersen WA, Bae R, et al. First experience with percutaneous mitral valve plication as primary therapy for symptomatic obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2016;67:2811-2818.
- Schäfer U, Frerker C, Thielsen T, et al. Targeting systolic anterior motion and left ventricular outflow tract obstruction in hypertrophic obstructed cardiomyopathy with a MitraClip. EuroIntervention. 2015;11:942-947.
- Schäfer U, Kreidel F, Frerker C. MitraClip implantation as a new treatment strategy against systolic anterior motion-induced outflow tract obstruction in hypertrophic obstructive cardiomyopathy. Heart Lung Circ. 2014:e131-e135.
- Rigopoulos AG, Seggewiss H. Twenty years of alcohol septal ablation in hypertrophic obstructive cardiomyopathy. Curr Cardiol Rev. 2016;12:285-296.
- Rastagan H, Boll G, Rowin EJ, et al. Results of surgical septal myectomy for obstructive hypertrophic cardiomyopathy: the Tufts experience. Ann Cardiothorac Surg. 2017;6:353-363.
- Gersh BJ, Maron BJ, Bonow RO, et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e212-e260. Epub 2011 Nov 8.
- Sorajja P, Brinder K, Richard B, et al. Maneuvers for technical success with transcatheter mitral valve repair. Catheter Cardiovasc Interv. 2018;92:617-626. Epub 2017 Feb 1.