Treating Peripheral Arterial Disease Percutaneously with Atherectomy
Abstract: Purpose. To determine clinical outcomes of patients who underwent percutaneous revascularization (PR) with multiple devices. Methods. PR cases at a private, tertiary referral hospital were reviewed retrospectively. Limb salvage and patency rates were calculated by the Kaplan Meier method. Historical and procedural factors were analyzed by multivariate Cox proportional hazards models. Results. We identified 66 patients and 87 limbs with 261 lesions, including 38 patients with critical limb ischemia (CLI) (51 limbs, 171 lesions). PR incorporated multiple devices (2.0 ± 1.2 devices/lesion, 2.4 ± 1.6 devices/procedure), including balloon angioplasty (57%), excisional atherectomy (54%), orbital atherectomy (44%), and stenting (13%). Last imaged patency was at 18 ± 13 months and last clinical follow-up was 22 ± 12 months. Thirty-five of 87 limbs had ≥1 repeat PR. In 51 limbs with CLI, limb salvage was 75% at 3 years. Independent predictors of amputation were higher creatinine (P=.01; hazard ratio [HR], 1.4), Rutherford category (P=.03; HR, 3.5), and history of coronary artery bypass graft (CABG) (P=.03; HR, 8.9). Overall patency remained 75% through 3 years. Loss of patency or primary patency (patency without repeat PR) was predicted by higher creatinine, Rutherford category, chronic total occlusion, history of CABG, female gender, current and past smoking. Use of excisional atherectomy maintained overall patency (P=.01; HR, 0.36). Conclusions. An aggressive approach to PR with frequent use of atherectomy resulted in high rates of limb salvage and patency. Smoking cessation and excisional atherectomy may improve patency rates.
J INVASIVE CARDIOL 2012;24(6):263-269
Key words: atherectomy, critical limb ischemia, peripheral vascular disease, peripheral interventions
_________________________________________
Peripheral arterial disease (PAD) can cause claudication that limits mobility. Critical limb ischemia (CLI), the most severe form of PAD, causes pain at rest in addition to nonhealing wounds or ulcers and in some cases necessitates amputation. Patients with CLI have significant cardiovascular comorbidities and a risk of mortality at 1 year of 25% and at 3 years of 50%.1-5 Since primary amputation for CLI is associated with a peri-operative mortality rate of 13%-17%, increased mortality compared with revascularization, and less than half of all patients ever achieving full mobility,1,6 primary revascularization has become the preferred treatment for patients with CLI.1-3,5,7 There has been only one randomized trial, with limited methodology, comparing outcomes between peripheral bypass surgery and percutaneous revascularization (PR), which showed no difference in survival at 1 year or 3 years, a significantly higher rate of early morbidity in the surgery group, a trend toward improved amputation-free survival after 2 years for the surgery group, and significantly reduced cost in the PR group.8 Because PR has demonstrated patency rates and limb salvage rates similar to bypass surgery (bypass surgery has better 1-year patency rates), and PR is associated with reduced early morbidity and does not interfere with the ability to perform subsequent procedures (if stents are avoided in the distal target zone), the literature has suggested an aggressive approach to revascularization via PR.1-3,7 Despite evidence supporting an aggressive approach to revascularization, primary amputation rates remain high and the use of vascular imaging for patients with CLI remains low.1 There have been numerous reports describing individual PR devices,3 but reports describing combinations of devices are limited,7 and comparative trial data are lacking. Therefore, the goal of this research project was to investigate clinical outcomes associated with an aggressive approach to revascularization using multiple devices for patients with PAD, including patients with CLI.
Methods
Approval was obtained from the Western IRB for this retrospective study. Patients treated between 1/1/07 and 12/31/08 were identified from a file generated by cardiovascular fellows who were instructed to complete a 1-page data form for each PR. The electronic medical record was used to collect data regarding patient demographics, medical history, clinical presentation, and clinical outcomes. Angiograms were reviewed in order to describe the lesion, procedural characteristics, the indication for each device, and to assess for angiographic success. Patients were included if they had >6 months of clinical follow-up, or if within 6 months of the intervention they underwent bypass surgery, a major amputation, or died. Patients were excluded if they had <6 months of clinical follow-up unless they had bypass surgery, a major amputation, or death. Interventions for infrainguinal atherosclerotic disease were included (from the femoral through the dorsalis pedis arteries). Iliac lesions were excluded because routine stenting is recommended in the iliac arteries, whereas provisional stenting is recommended in the infrainguinal arteries.
Definitions. Patients were stratified by Rutherford category,9 and categories 4-6 were considered CLI. Limb salvage was defined as the absence of a major amputation on the revascularized limb, including high forefoot amputations or larger, but not including minor amputations of the toe or metatarsal zones. Angiographic success was defined as <30% residual stenosis by visual estimation. In-line flow was angiographic success with continuous flow from the iliac to the dorsalis pedis via the anterior tibial artery or to the plantar artery via the posterior tibial artery. Failures were limbs in which lesions failed to be crossed with a wire (ie, no intervention could be attempted). Patency required verification by angiography, Duplex ultrasound imaging, or magnetic resonance imaging. Lesions and limbs were considered primarily patent if continuous flow was uninterrupted without any additional procedures on the lesion or limb to maintain patency. If lesions or limbs remained patent after undergoing repeat PRs, they were classified as having assisted primary patency or secondary patency. If patency was lost (total occlusion) and then restored, the lesion or limb was considered secondarily patent. Assisted primary patency was defined as patency that was never lost (non-occluded restenosis), but required repeat PR to maintain patency. Clinical improvement was defined as an improvement in Rutherford category with one of the following: (1) improvement in ankle-brachial index (or toe-brachial index) of >0.10; (2) healing of a previously nonhealing wound; or (3) for patients without a baseline ankle-brachial index, a postprocedure ankle-brachial index of >0.50 for patients with CLI or >0.9 for patients with baseline Rutherford class 0-3.
Therapy and percutaneous revascularization. All clinical decisions were at the discretion of the attending physicians, which generally include the principles described as follows. Patients with severe PAD underwent non-invasive ultrasound imaging of the peripheral arteries before proceeding to angiography. Consultation with a vascular surgeon was obtained for patients with advanced disease. Revascularization was attempted prior to amputation. PR was the preferred initial approach; bypass surgery was considered as a second revascularization option and recommended to all patients prior to amputation.
During the percutaneous procedure, patients were anticoagulated with heparin to maintain a procedural activated clotting time of 200-300 seconds. Arterial access was achieved with 5-7 Fr sheaths, usually via contralateral retrograde femoral access, with either 45-cm or 90-cm long sheaths. Multiple 0.035˝, 0.018˝, and 0.014˝ wires were utilized, often in combination to cross difficult chronic total occlusions (CTOs). Specialized interventional devices were selected depending upon lesion characteristics and the anatomic level of disease. After the procedure, all patients were placed on both aspirin and clopidogrel unless there were contraindications to anti-platelet therapy.
PR was performed using multiple devices: 2.0 ± 1.2 devices/lesion, and 2.4 ± 1.6 devices/procedure. Device use included balloon angioplasty in 57%, SilverHawk Plaque Excision System (ev3 Endovascular, Inc.) in 54%, Diamondback 360° PAD System (Cardiovascular Systems, Inc.) in 44%, and stenting in 13%. The Crosser Catheter (Bard Peripheral Vascular Inc.) was successful in 13 of 18 attempts (72%) at crossing a CTO. A Spider Embolic Protection Device (ev3 Endovascular, Inc.) was used in conjunction with SilverHawk atherectomy in 10 limbs (11%) and once because of a high thrombus burden. Acute ischemia due to thrombus was treated primarily with the Angiojet Thrombectomy System (MEDRAD, Inc.), with occasional use of tissue plasminogen activator infusion and systemic heparin. Balloon angioplasty was utilized as an initial device for predilation (7%) and focal lesions (5%), and was used at the conclusion of the procedure for stent postdilation (6%) and to treat residual stenosis (20%), dissections, perforations, and severe spasm. A cutting balloon was used sparingly for calcified lesions, predilation, a CTO, and for fibrotic disease in a vein graft. Atherectomy was the primary treatment modality in this series with SilverHawk and Diamondback atherectomy each used in approximately half of the patients. SilverHawk atherectomy was employed in areas of flexion (12%), for long diffuse disease (35%), for bifurcation lesions (3%), for residual stenosis (3%), operator preference (5%), and once each to treat a dissection and for in-stent restenosis. Diamondback atherectomy was used primarily for calcified lesions (36%) and for long tibial CTOs (7%).10 Stenting was performed provisionally for residual disease (4%), dissection, perforation, and severe spasm.
Following PR, patients underwent a clinical assessment with non-invasive ultrasound imaging every 3 months for the first year, then every 6 months, or earlier if symptoms worsened.
 Statistics. Data are presented as a total cohort and separately for patients with CLI. Kaplan-Meier estimates11 were used to derive limb salvage and patency rates by intention-to-treat (ie, including failed procedures). Fifteen historical variables and 7 procedural factors were first analyzed by a univariate Cox proportional hazards model12 to identify factors associated with amputation and patency. Then, factors with P<.20 were analyzed in multivariate models to identify independent predictors of amputation, patency, and primary patency. A P-value <.05 was considered significant in the multivariate models. Results are reported as a hazard ratio (HR). A hazard ratio >1.0 indicates increased risk of losing patency. An HR <1.0 indicates the variable preserved patency. Failures (5 limbs, 16 lesions) were excluded from the Cox models so that the effects of PR treatment modalities could be assessed. Limbs (3) and lesions (4) with undocumented patency during follow-up were excluded from the Cox models for patency. Of the 3 limbs without documented patency, 1 had healing of a previously non-healing wound and 2 had no change in their Rutherford category at final follow-up (Rutherford 4).
Statistics. Data are presented as a total cohort and separately for patients with CLI. Kaplan-Meier estimates11 were used to derive limb salvage and patency rates by intention-to-treat (ie, including failed procedures). Fifteen historical variables and 7 procedural factors were first analyzed by a univariate Cox proportional hazards model12 to identify factors associated with amputation and patency. Then, factors with P<.20 were analyzed in multivariate models to identify independent predictors of amputation, patency, and primary patency. A P-value <.05 was considered significant in the multivariate models. Results are reported as a hazard ratio (HR). A hazard ratio >1.0 indicates increased risk of losing patency. An HR <1.0 indicates the variable preserved patency. Failures (5 limbs, 16 lesions) were excluded from the Cox models so that the effects of PR treatment modalities could be assessed. Limbs (3) and lesions (4) with undocumented patency during follow-up were excluded from the Cox models for patency. Of the 3 limbs without documented patency, 1 had healing of a previously non-healing wound and 2 had no change in their Rutherford category at final follow-up (Rutherford 4).
Results
 We identified 66 patients and 87 limbs with 261 lesions, including 38 patients with CLI (51 limbs, 171 lesions) (Table 1). Patients were elderly (mean age, 72.4 ± 11.5 years). Cardiovascular comorbidities were prevalent, including hypertension in 82%, diabetes mellitus in 56% (32% insulin-dependent), renal insufficiency in 36%, and a history of coronary artery bypass graft surgery in 21%. A CTO was present in 45% of limbs with CLI and in 12 of 36 limbs (33%) with a Rutherford category 0-3 (Table 2). Twenty-six percent of all lesions were CTOs and 68% were moderate-severely calcified. The superficial femoral artery was intervened upon most often overall, with a shift toward more infrapopliteal disease in patients with CLI.
We identified 66 patients and 87 limbs with 261 lesions, including 38 patients with CLI (51 limbs, 171 lesions) (Table 1). Patients were elderly (mean age, 72.4 ± 11.5 years). Cardiovascular comorbidities were prevalent, including hypertension in 82%, diabetes mellitus in 56% (32% insulin-dependent), renal insufficiency in 36%, and a history of coronary artery bypass graft surgery in 21%. A CTO was present in 45% of limbs with CLI and in 12 of 36 limbs (33%) with a Rutherford category 0-3 (Table 2). Twenty-six percent of all lesions were CTOs and 68% were moderate-severely calcified. The superficial femoral artery was intervened upon most often overall, with a shift toward more infrapopliteal disease in patients with CLI.
 Procedural and 30-day clinical outcomes. In limbs with Rutherford category 0-3, 100% of procedures achieved in-line flow (Table 3). In limbs with CLI, angiographic success was achieved in 80% and in-line flow in 75%; 10% were failures. The incidence of procedural complications was low, including dissection in 3%, perforation in 0%, embolization in 5%, and major bleeding in 1%. No patient developed a peri-procedural myocardial infarction. One patient died within 30 days after undergoing bypass surgery and a minor amputation. Within 30 days, 8% of patients with CLI underwent a minor amputation and 4% had a major amputation, whereas 33% were initially categorized as Rutherford 6 and were anticipated to require an amputation.
Procedural and 30-day clinical outcomes. In limbs with Rutherford category 0-3, 100% of procedures achieved in-line flow (Table 3). In limbs with CLI, angiographic success was achieved in 80% and in-line flow in 75%; 10% were failures. The incidence of procedural complications was low, including dissection in 3%, perforation in 0%, embolization in 5%, and major bleeding in 1%. No patient developed a peri-procedural myocardial infarction. One patient died within 30 days after undergoing bypass surgery and a minor amputation. Within 30 days, 8% of patients with CLI underwent a minor amputation and 4% had a major amputation, whereas 33% were initially categorized as Rutherford 6 and were anticipated to require an amputation.
Repeat procedures. Repeat PRs were performed in 35 of 87 limbs (40%) overall and in 22 of 51 limbs with CLI (43%). Repeat PRs tended to occur early (≤5 months in 57%), or late (≥12 months in 29% at a mean of 19.7 ± 4.6 months). Of all 87 limbs, 25% had only 1 repeat PR, 7% had 2 repeat PRs, 5% had 3 repeat PRs, and 3% had >3 repeat PRs. Repeat PRs were indicated for restenosis (18%), de novo lesions (8%), and for combined restenosis and de novo lesions (14%).
Clinical outcomes. Last clinical follow-up was at a mean of 22.4 ± 12.1 months. Of the 51 patients with CLI, after the index procedure, clinical improvement was demonstrated in 31 (61%), 6 (12%) remained in the same Rutherford category, and 18 of 35 wounds healed (51%). At last follow-up, 58% of wounds healed, 53% had clinical improvement, and an additional 14% remained clinically the same without a major amputation (Table 4). In the 51 limbs with CLI, limb salvage was achieved in 38 limbs (75%) at 3 years (Figure 1). Twelve of 13 major amputations (92%) occurred within the first 7 months. Independent predictors of amputation were higher creatinine (P=.011; HR, 1.4), Rutherford category (P=.026; HR, 3.5), and history of coronary artery bypass graft surgery (P=.026; HR, 8.9).
 Clinical outcomes correlated with limb patency (Table 4). Patients without angiographic success on the index procedure had poor outcomes. Of patients without angiographic success who did not undergo subsequent bypass surgery, only 2 of 7 (29%) had wound healing and 5 of 8 (62%) had a major amputation at a mean of 3.2 months. Two patients without angiographic success underwent subsequent bypass surgery; 1 had wound healing, 1 had a major amputation at 3.4 years.
Clinical outcomes correlated with limb patency (Table 4). Patients without angiographic success on the index procedure had poor outcomes. Of patients without angiographic success who did not undergo subsequent bypass surgery, only 2 of 7 (29%) had wound healing and 5 of 8 (62%) had a major amputation at a mean of 3.2 months. Two patients without angiographic success underwent subsequent bypass surgery; 1 had wound healing, 1 had a major amputation at 3.4 years.
 Patency. Last imaged patency was at a mean of 18.0 ± 12.6 months (Figure 1). Primary patency decreased to 50% at 1 year and 36% at 2 years. With repeat PR, overall patency was maintained at 75% through 3 years. Most limbs that lost overall patency underwent major amputation within the first 7 months.
Patency. Last imaged patency was at a mean of 18.0 ± 12.6 months (Figure 1). Primary patency decreased to 50% at 1 year and 36% at 2 years. With repeat PR, overall patency was maintained at 75% through 3 years. Most limbs that lost overall patency underwent major amputation within the first 7 months.
Independent predictors of loss of patency (or maintenance of patency) are shown in Table 5. Loss of both primary patency and overall patency was predicted by higher Rutherford category, CTO, and current smoking. Female gender and former smoking predicted loss of primary patency, but not overall patency. Higher creatinine and a previous coronary artery bypass graft surgery predicted loss of patency. Surprisingly, factors that predicted maintenance of patency were a history of hypercholesterolemia and moderate-severe calcification. A “history of hypercholesterolemia” represented patients being treated for hypercholesterolemia and was a marker for statin therapy (use of statins at baseline in patients with versus without a history of hypercholesterolemia: 28 of 36 [78%] versus 1 of 30 [3%]). Use of SilverHawk atherectomy predicted maintenance of overall patency, but not primary patency.
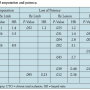 On multivariate analysis of the overall cohort, the presence of moderate-severe calcification preserved primary patency (P=.0123; HR, 0.5887). When analyzing only lesions with moderate-severe calcification, use of Diamondback atherectomy maintained primary patency on univariate analysis (P=.0195; HR, 0.5958), but not on multivariate analysis (P=.1533; HR, 0.6541).
On multivariate analysis of the overall cohort, the presence of moderate-severe calcification preserved primary patency (P=.0123; HR, 0.5887). When analyzing only lesions with moderate-severe calcification, use of Diamondback atherectomy maintained primary patency on univariate analysis (P=.0195; HR, 0.5958), but not on multivariate analysis (P=.1533; HR, 0.6541).
Discussion
The main findings of this analysis are that in a private hospital, an aggressive approach to PR using multiple devices with frequent use of atherectomy devices salvaged 75% of limbs with CLI and maintained overall patency in 75% through 3 years. Predictors of loss of patency were also identified. Modifiable factors that maintained patency were identified, including smoking cessation, statin therapy, and use of SilverHawk atherectomy.
Clinical outcomes. PR was attempted in nearly all patients with CLI in this institution, despite severe disease as indicated by the high prevalence of CTOs (40% of all limbs). This approach maintains all therapeutic options during follow-up and avoids surgical risks in these patients with severe cardiovascular comorbidities. In the bypass surgery arm of the BASIL trial,8 which used extensive exclusion criteria, the incidence of perioperative myocardial infarction was 13 of 197 (7%) and 30-day mortality was 5%. In this “real-world” experience of all patients who underwent PR for PAD, no patients suffered periprocedural myocardial infarction and only 1 patient (1%) died after subsequently undergoing bypass surgery and a minor amputation.
All but 1 amputation and most losses of overall patency occurred in the first 7 months after the index procedure, probably reflecting disease that was not amendable to percutaneous or surgical revascularization. In particular, limbs without angiographic success had poor outcomes (Table 4). In contrast, the 2 limbs without angiographic success that underwent early bypass surgery had better outcomes: 1 limb had wound healing and 1 had a major amputation delayed 3.4 years. However, the only patient who died within 30 days had undergone bypass surgery (despite angiographic success on the index procedure). In the BASIL trial,8 bypass surgery tended to improve amputation-free survival after 6 months relative to PR, but bypass surgery increased early morbidity and tended to increase early mortality. Thus, a lack of angiographic success during PR may indentify a subset of patients in which bypass surgery should be considered after careful risk-benefit assessment.
Although primary patency continued to decrease beyond 7 months, patency was maintained with repeat PR, highlighting the benefit of initial PR to leave options available for later revascularization.
Cost comparison. Cost analyses comparing primary amputation, bypass surgery and PR for patients with PAD are challenging and depend on the patient population and on the cost of the initial procedure, of hospital days, of complications and of repeat procedures. An original cost analysis and review of the literature1 concluded that both bypass surgery and PR are cost effective compared with primary amputation because primary amputation necessitates prolonged hospitalization, prolonged nursing home living, and prolonged rehabilitation in many patients because the majority of patients never achieve full mobility. Comparing bypass surgery with PR is more difficult. The aforementioned analysis1 describes similar costs between the two modalities, slightly favoring PR. The BASIL trial,8 which randomized patients to bypass surgery or PR, reported that costs for bypass surgery were approximately a third higher than PR costs through the first year, due largely to longer inpatient hospital stays in the bypass surgery arm. In general, the cost of the initial hospital stay and complications are higher for bypass surgery and the cost of repeat procedures is higher for PR. Costs for the initial procedure were considerably higher for this series than in the BASIL trial, because this series utilized atherectomy devices (whereas the BASIL trial featured balloon angioplasty and provisional stenting). The relatively high repeat procedure rate observed in this series would be associated with increased costs over time.
Atherectomy. The landmark publications regarding PR for CLI (BASIL8 and TASC5) were based on balloon angioplasty, not atherectomy. Thus, this analysis of “real world” patients treated with multiple devices and predominantly with atherectomy updates the current literature. Patency rates reported here are similar to those reported in several SilverHawk atherectomy series13-15 and slightly inferior to a Diamondback series with a less severely diseased patient population.10,16 The finding that treatment with SilverHawk atherectomy did not impact primary patency, but preserved overall patency on multivariate analysis, supports the concept of using atherectomy to keep PR options open during follow-up. In vessels with long, diffuse disease, plaque removal may be advantageous over balloon angioplasty, which disrupts the intima and displaces plaque within the lumen. Lesion length was an important predictor of patency following balloon angioplasty,5 but did not predict patency in this or in another series15 of limbs treated predominantly with SilverHawk atherectomy, supporting its use in long, diffusely diseased vessels.
Traditionally, heavily calcified lesions are notoriously difficult to manage percutaneously and have historically high rates of restenosis. Intriguingly, the presence of moderate-severe calcification predicted maintenance of primary patency in this series in which calcified lesions were treated primarily with Diamondback atherectomy. Several possible mechanisms may explain this phenomenon. If the calcified atheroma can be adequately debulked and the vessel recanalized, the calcium remaining in the vessel wall may theoretically act as a barrier to neointimal hyperplasia and smooth muscle cell migration. In support of this concept, an intravascular ultrasound study reported significantly less neointimal hyperplasia, less cross-sectional narrowing, and increased lumen area in calcified versus less-calcified vessel segments 6 months after bare-metal stent implantation.17 Alternatively, the remaining calcified plaque may act as a “natural stent” keeping the vessel patent.
Predictors of clinical outcomes. Higher creatinine level predicted amputation, probably because renal insufficiency is a cardiovascular risk factor and because repeat PR may have been avoided and contrast use minimized in patients with elevated creatinine. A history of coronary artery bypass graft surgery is a marker of longstanding atherosclerosis and predicted amputation with a sizable HR of 8.9. Moreover, patients with a history of coronary artery bypass grafting may have lacked adequate vein conduits, precluding bypass surgery and instead steering these patients toward PR or amputation. For the same reasons, higher creatinine and a history of coronary artery bypass graft surgery predicted loss of patency, along with Rutherford category, which is a measure of the severity of PAD.
The presence of a CTO, perhaps the best marker of severe local disease, consistently predicted loss of primary patency and overall patency with HRs of 2.5 to 2.9. Female gender is known to increase restenosis rates related to smaller vessel diameter, which was not measured. Interestingly, female gender did not predict overall patency or amputation, suggesting that patency in females could be reestablished with repeat PR.
Current smoking consistently predicted loss of patency with HRs of 2.7 to 2.9. In contrast, past smoking only predicted loss of primary patency in one model with a HR of only 1.7. These results are in accordance with a recent study18 of nearly 40,000 women that reported current smoking to be a potent risk factor and former smoking to be a mild risk factor for symptomatic PAD. These reports support the role of smoking cessation to reduce the risk of developing new or recurrent severe PAD.
In patients with PAD, the use of statins reduces the risk of major adverse cardiovascular events5 and appeared to improve functional status and 6-minute walk performance compared to PAD patients not taking statins in two retrospective analyses.19,20 In this study, statins appeared to improve patency rates through an indirect measure of a “history of hypercholesterolemia,” which was a marker for statin therapy use at baseline.
In patients with PAD, it is well known that the presence of diabetes mellitus predicts worse outcomes for patency, limb salvage, and overall survival.5,8,15 In this series, diabetes did not significantly increase the risk of losing patency in any model. This concurs with two other reports on SilverHawk atherectomy in which diabetes did not increase target lesion revasularization rates.14,21 Perhaps patients with diabetes have better outcomes with atherectomy compared with balloon angioplasty and provisional stenting or perhaps some anti-diabetic medicines may have a protective effect on the vasculature.
Medications. Table 1 shows the incidences of prescription drug use upon presentation to the interventional cardiologist for symptoms of PAD. Nearly all patients were previously evaluated by a primary care physician, most had known atherosclerosis, and all had suspected PAD. Considering the evidence supporting the use, in high-risk patients, of statins5 and anti-platelet agents (aspirin and clopidogrel),22 use of these drugs in this cohort with known or suspected PAD should ideally have been near 100%. Suboptimal use of these medications highlights an area with the potential to improve outcomes in patients with PAD: improved education of primary care providers on the beneficial impact of medications in patients with PAD and methods to improve compliance with prescription drug use.
Future studies. The hypotheses generated here require confirmation in randomized trials. Specifically, the following topics warrant further investigation:
- Comparison of bypass surgery to PR using multiple devices and predominantly atherectomy devices with clinical endpoints, including survival, amputation-free survival, and major adverse cardiovascular events. eV3 designed the PROOF study in 2007 in hopes of answering this question, but patient enrollment proved very challenging.
- Comparison of outcomes in patients with PAD and diabetes undergoing atherectomy versus balloon angioplasty with provisional stenting.
- Comparison of SilverHawk atherecomy versus balloon angioplasty with provisional stenting for all patients with PAD.
- Comparison of Diamondback atherectomy versus balloon angioplasty with provisional stenting for calcified peripheral lesions (CALCIUM 360° Registry currently underway).
- Comparison of SilverHawk atherectomy versus Diamondback atherectomy for all patients with PAD.
- Comparison of restenosis and revascularization rates for PAD in patients with versus without statins.
Limitations. As a retrospective analysis in a single center, it is uncertain how these results would translate prospectively to the interventional community. Operators had a low threshold for attempting PR even in patients with severe multilevel disease with the goal of avoiding major amputation. More strict selection of patients may have resulted in improved outcomes. To reflect real-world experience, this analysis did not restrict patient enrollment, so it is difficult to compare these results to trials with exclusion criteria. Recruitment of patients from a file generated by fellows resulted in a nonconsecutive sample with bias toward including patients who underwent multiple PRs and may have inflated the reported incidence of repeat PRs. Due to the retrospective nature of data collection, deaths occurring outside of the hospital may have been missed, so data analysis focused on limb salvage and patency rates similar to previous publications.13-15 Thus, limb salvage reflects the absence of a major amputation in surviving patients. Patency rates reflect the patency at last imaging. This study did not systematically compare treatment modalities; therefore, the results are hypothesis-generating and require confirmation in randomized trials.
Conclusion
PR with multiple devices with a bias toward atherectomy devices can be performed in a private hospital setting with high procedural success and minimal early morbidity and mortality. This approach salvaged 75% of limbs with CLI through 3 years. PR with atherectomy preserves options for future revascularization. Overall patency was maintained in 75% with repeat PR. Smoking cessation, statins, and use of SilverHawk atherectomy may improve patency rates.
Acknowledgments. We greatly appreciate the statistical tools that John Pezzullo made available on his webpage at www.statpages.org. Sincere thanks to Ryan Bhandari for help with data collection.
References
- Allie DE, Hebert CJ, Lirtzman MD, et al. Critical limb ischemia: a global epidemic a critical analysis of current treatment unmasks the clinical and economic costs of CLI. EuroIntervention. 2005;1(1):60-69.
- Slovut DP, Sullivan TM. Critical limb ischemia: medical and surgical management. Vasc Med. 2008;13(3):281-291.
- Arain SA, White CJ. Endovascular therapy for critical limb ischemia. Vasc Med. 2008;13(3):267-279.
- Wolfe JH, Wyatt MG. Critical and subcritical ischaemia. Eur J Vasc Endovasc Surg. 1997;13(6):578-582.
- Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33(Suppl 1):S1-S70.
- The Vascular Surgical Society of Great Britain and Ireland. Critical limb ischaemia: management and outcome. Report of a national survey. Eur J Vasc Endovasc Surg. 1995;10(1):108-113.
- Shah AP, Klein AJ, Sterrett A, et al. Clinical outcomes using aggressive approach to anatomic screening and endovascular revascularization in a veterans affairs population with critical limb ischemia. Catheter Cardiovasc Interv. 2009;74(1):11-19.
- Adam DJ, Beard JD, Cleveland T, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366(9501):1925-1934.
- Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26(3):517-538.
- Safian RD, Niazi K, Runyon JP, et al. Orbital atherectomy for infrapopliteal disease: device concept and outcome data for the OASIS trial. Catheter Cardiovasc Interv. 2009;73(3):406-412.
- Kaplan-Meier survival plot with 95% confidence interval and logrank test. Technical University of Denmark 2011; Available at: https://iscc-serv2.imm.dtu.dk/~merser/K-M_plot.php.
- Pezzullo JC. Cox proportional hazards survival regression. Georgetown University 2011; Available at: https://www.statpages.org/prophaz.html.
- Keeling WB, Shames ML, Stone PA, et al. Plaque excision with the SilverHawk catheter: early results in patients with claudication or critical limb ischemia. J Vasc Surg. 2007;45(1):25-31.
- Ramaiah V, Gammon R, Kiesz S, et al. Midterm outcomes from the TALON registry: treating peripherals with SilverHawk: outcomes collection. J Endovasc Ther. 2006;13(5):592-602.
- McKinsey JF, Goldstein L, Khan HU, et al. Novel treatment of patients with lower extremity ischemia: use of percutaneous atherectomy in 579 lesions. Ann Surg. 2008;248(4):519-528.
- Weinstock B, Dulas D, Runyon JP, et al. Durable results found in patients followed more than two years post orbital atherectomy treatment (Abstr). Am J Cardiol. 2009;104:214D.
- Shimada Y, Kataoka T, Courtney BK, et al. Influence of plaque calcium on neointimal hyperplasia following bare metal and drug-eluting stent implantation. Catheter Cardiovasc Interv. 2006;67(6):866-869.
- Conen D, Everett BM, Kurth T, et al. Smoking, smoking status, and risk for symptomatic peripheral artery disease in women: a cohort study. Ann Intern Med. 2011;154(11):719-726.
- McDermott MM, Guralnik JM, Greenland P, et al. Statin use and leg functioning in patients with and without lower-extremity peripheral arterial disease. Circulation. 2003;107(5):757-761.
- Giri J, McDermott MM, Greenland P, et al. Statin use and functional decline in patients with and without peripheral arterial disease. J Am Coll Cardiol. 2006;47(5):998-1004.
- Kandzari DE, Kiesz RS, Allie D, et al. Procedural and clinical outcomes with catheter-based plaque excision in critical limb ischemia. J Endovasc Ther. 2006;13(1):12-22.
- CAPRIE steering committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348(9038):1329-1339.
_________________________________________
From the 1Heart Institute, Good Samaritan Hospital, Los Angeles, California, 2Department of Internal Medicine, Division of Cardiovascular Medicine, Keck School of Medicine at the University of Southern California, Los Angeles, California, and the 3Department of Cardiology, Good Samaritan Hospital, Los Angeles, California.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Burstein is on the Abbott Vascular Speaker’s Bureau. Dr Mayeda has a training, research and consulting agreement with ev3 Endovascular, Inc. and Cardiovascular Systems, Inc. He is an investor with Cardiovascular Systems, Inc and is on the Medtronic Speaker’s Bureau. No other authors report disclosures.
Manuscript submitted October 18, 2011, provisional acceptance given January 2, 2012, final version accepted February 8, 2012.
Address for correspondence: Bryan G. Schwartz, MD, Heart Institute, Good Samaritan Hospital 1225 Wilshire Blvd, Los Angeles, CA 90017-2395. Email: bschwartz15@hotmail.com
















