Transfemoral Aortic Valve-in-Valve Implantation With the CoreValve Evolut for Small Degenerated Stented Bioprosthesis
Abstract: Transcatheter aortic valve-in-valve implantation represents one interesting therapeutic option for high-risk surgical patients with degenerated bioprostheses. The procedure is less invasive and can be performed without thoracotomy and general anesthesia, if the femoral approach is used. Until recently, failing small bioprostheses could only be treated percutaneously by underexpanding the CoreValve (Medtronic, Inc) or Edwards Sapien valve (Edwards Lifesciences). Underexpansion of these valves might compromise the hemodynamic performance and potentially limit its durability. Herein, we report our initial experience with the 23 mm CoreValve Evolut in 4 patients with degenerated 21 mm Mitroflow valves. The CoreValve prosthesis was successfully implanted in all 4 patients, with no major complications and no mortality at 3-month follow-up exam. However, 2 of the 4 patients developed mildly elevated transvalvular gradients. Therefore, despite our promising results, caution is necessary when considering patients with small degenerated bioprostheses for a valve-in-valve procedure.
J INVASIVE CARDIOL 2014;26(6):291-294
Key words: transcatheter aortic valve implantation, bioprosthesis, aortic valve disease
____________________________
For patients with severe aortic stenosis or regurgitation, surgical aortic valve replacement is usually the treatment of choice. Mechanical prostheses are very durable, but require life-long anticoagulation therapy. During recent years, the use of bioprostheses has become more common,1 as they do not require anticoagulation and subsequent models are expected to show increased durability. Despite this, tissue degeneration is the main cause of bioprosthetic failure resulting in stenosis, regurgitation, or combined failure and may require reoperation. In a long-term follow-up study of 1119 patients, Glower et al2 reported freedom from reoperation of patients for a failing bioprosthetic valve of 95%, 90%, and 70% at 5, 10, and 15 years, respectively. Redo surgery in older patients with comorbidities for degenerated bioprostheses is technically more demanding and associated with a higher mortality and morbidity. Transcatheter aortic valve-in-valve implantation (ViV-TAVI) is an alternative therapeutic option for these patients. Two established TAVI systems have found broad clinical use for such procedures, such as the balloon-expandable Edwards Sapien valve, and the self-expandable Medtronic CoreValve system (MCV). Both transcatheter valves have been used effectively for ViV therapy. However, degenerated bioprostheses with a small diameter (<21 mm) represent a technical and anatomical challenge for ViV therapy. Here, we report our first 4 cases of degenerated 21 mm Mitroflow bioprostheses managed with the 23 mm CoreValve Evolut.
Methods
Patient cohort. From a total number of 640 TAVI cases at our institution, we included 4 consecutive symptomatic patients with failing 21 mm Mitroflow bioprosthesis because of stenosis (n = 1), regurgitation (n = 1), or combined failure (n = 2) who were at high risk (logistic EuroSCORE >20%) for surgical aortic valve redo surgery. All cases were discussed and the indications approved by the institutional heart team. Femoral access vessel size was at least 6 mm in all patients and therefore suitable for transfemoral approach with CoreValve implantation (18 Fr).
Procedure. ViV implantation was performed with the 23 mm Medtronic CoreValve Evolut in all patients, as it is particularly suitable for small native annular sizes between 18-20 mm. The procedure was performed under conscious sedation and local anesthesia. After placing a preclosure device (Prostar XL; Abbott Vascular), an 18 Fr Cook sheath was introduced into the right common femoral artery. After passing the failing Mitroflow prosthesis with a straight-tip Terumo wire, this was exchanged to an Amplatzer superstiff wire (Boston Scientific) advanced into the left ventricle. Balloon valvuloplasty was not performed to avoid debris embolization, deformation of the stent frame, and the risk of stent fracture. The MCV Evolut was deployed under fluoroscopic control. Aortic and ventricular pressures as well as transvalvular gradient were measured before and immediately after ViV implantation. Procedural success and major clinical endpoints were assessed in accordance with the Valve Academic Research Consortium-2 (VARC-2) criteria.3
Follow-up. Predischarge transthoracic echocardiography was performed on days 4-6 after MCV Evolut implantation. All 4 patients completed follow-up to 3 months at our outpatient department.
Results
All 4 patients had failing 21 mm Mitroflow prostheses, were 87 ± 1.3 years old, and showed an average logistic EuroSCORE of 37 ± 10% and mean STS score of 8.6 ± 2.5%. Stenotic valve degeneration was present in the majority of patients (n = 3), whereas 1 patient showed high-grade aortic regurgitation of the implanted Mitroflow prosthesis. Table 1 shows the detailed baseline characteristics of the 4 patients. All patients were treated with 23 mm MCV Evolut implantation and the MCV could be deployed successfully in all patients. Table 2 summarizes the periprocedural characteristics and results.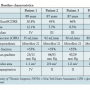 One patient required a pacemaker implantation due to complete atrioventricular block, and 1 minor vascular access complication (failure of preclosure device managed with implantation of an endovascular stent-graft) occurred. There was no stroke or coronary obstruction. According to the VARC-2 definition, device failure was present in 2 patients (50%) because of mildly elevated postprocedural invasive gradients. However, the VARC definition was originally intended for TAVI procedures in native aortic valves and not for ViV therapy.
One patient required a pacemaker implantation due to complete atrioventricular block, and 1 minor vascular access complication (failure of preclosure device managed with implantation of an endovascular stent-graft) occurred. There was no stroke or coronary obstruction. According to the VARC-2 definition, device failure was present in 2 patients (50%) because of mildly elevated postprocedural invasive gradients. However, the VARC definition was originally intended for TAVI procedures in native aortic valves and not for ViV therapy.
All 4 patients were alive at 3 months, and no patient was lost to follow-up. All patients reported clinical improvement and symptom relief. According to New York Heart Association classification, 1 patient improved from class IV to III and the other 3 patients improved from class III to II. There were no signs of hemolysis. Echocardiography showed mildly elevated mean transvalvular gradients in 2 patients. No other adverse events were reported during follow-up.
Discussion
Transcatheter aortic valve implantation via the femoral route (TF-TAVI) is an option for patients with symptomatic aortic valve stenosis considered to be at high risk for conventional open-heart surgery.4,5 Elderly patients with degenerated bioprosthetic heart valves and/or significant comorbidities are a subgroup of patients in whom transcatheter valve-in-valve implantation is an interesting  option. Feasibility and effectiveness of the transapical and transfemoral approach were recently proven.6-8 However, smaller bioprostheses with an outer diameter of 19-21 mm are especially challenging because in these cases, ViV therapy is associated with excessive oversizing. Underexpansion of the transcatheter valve due to small dimensions of the degenerated bioprosthesis may result in unsatisfactory hemodynamics, significant residual transvalvular gradients, or transvalvular regurgitation due to potentially inadequate leaflet coaptation. In addition, Azadani et al9 have detected severe valve malfunction in an in vitro model of 19 and 21 mm bioprostheses after ViV implantation of a 23 mm Edwards Sapien valve was performed.9 While significant reduction of mean pressure gradient and total energy loss could be achieved with 23 mm bioprostheses, the effect was significantly reduced in 19 and 21 mm bioprostheses. Furthermore, incomplete stent expansion resulted in leaflet distortion and central regurgitation.
option. Feasibility and effectiveness of the transapical and transfemoral approach were recently proven.6-8 However, smaller bioprostheses with an outer diameter of 19-21 mm are especially challenging because in these cases, ViV therapy is associated with excessive oversizing. Underexpansion of the transcatheter valve due to small dimensions of the degenerated bioprosthesis may result in unsatisfactory hemodynamics, significant residual transvalvular gradients, or transvalvular regurgitation due to potentially inadequate leaflet coaptation. In addition, Azadani et al9 have detected severe valve malfunction in an in vitro model of 19 and 21 mm bioprostheses after ViV implantation of a 23 mm Edwards Sapien valve was performed.9 While significant reduction of mean pressure gradient and total energy loss could be achieved with 23 mm bioprostheses, the effect was significantly reduced in 19 and 21 mm bioprostheses. Furthermore, incomplete stent expansion resulted in leaflet distortion and central regurgitation.
Special aspects of the Mitroflow bioprosthesis. The Mitroflow bioprosthesis is a stented valve with a silicone base ring, an acetyl homopolymer stent, and leaflets manufactured from bovine pericardium. The fluoroscopic marker is located within the sewing ring at the base of the frame. A unique feature of the valve design is the fact that the leaflet tissue is mounted on the exterior side of the valve frame in order to maximize the effective orifice area. However, this also results in a higher potential risk of coronary obstruction during ViV-TAVI. The Mitroflow valve frame itself is not visible on  fluoroscopy. During surgical implantation, the sewing ring is sutured to the native aortic annulus in supra-annular position. This sewing ring becomes the anchor for ViV-TAVI implantation, thereby limiting the size of the transcatheter heart valve. The 21 mm Mitroflow bioprosthesis has an internal stent diameter of 17.3 mm, an outer stent diameter of 20.7 mm, an external sewing ring diameter of 23 mm, and a profile height of 13 mm (Figure 2). For ViV-TAVI, the internal stent diameter is the crucial parameter for the correct sizing of the transcatheter heart valve.
fluoroscopy. During surgical implantation, the sewing ring is sutured to the native aortic annulus in supra-annular position. This sewing ring becomes the anchor for ViV-TAVI implantation, thereby limiting the size of the transcatheter heart valve. The 21 mm Mitroflow bioprosthesis has an internal stent diameter of 17.3 mm, an outer stent diameter of 20.7 mm, an external sewing ring diameter of 23 mm, and a profile height of 13 mm (Figure 2). For ViV-TAVI, the internal stent diameter is the crucial parameter for the correct sizing of the transcatheter heart valve.
Potential risks of ViV-TAVI. The Edwards Sapien valve can be implanted into failed bioprostheses6 with good results through the apical approach. However, a 23 mm Edwards Sapien prosthesis requires a minimum inner stent diameter of 18.2 mm of the degenerated valve in order to reach full expansion, so in the case of a 21 mm Mitroflow, the inner stent diameter is not sufficiently large enough. In addition, in cases of failed Mitroflow bioprostheses, severe and fatal complications such as ostial coronary obstruction have been reported.10 This can occur as a consequence of relatively high radial forces on the stent frame of the Mitroflow prosthesis during balloon expansion of the Sapien valve, leading to deformation of the frame and possible coronary occlusion. The risk of this potentially fatal complication is further increased as a consequence of the valve design of the Mitroflow, because the leaflet tissue is mounted on the outer side of the frame. Thus, it is not surprising that the Global Valve-in-Valve Registry reports ostial coronary obstruction in 7.7% of the overstented Mitroflow prostheses, which is significantly higher than in any other stented bioprosthesis.11 Due to the slim stent design and the shape of the CoreValve Evolut, the risk of coronary obstruction is considerably lower and no ostial coronary obstruction was observed in the treated patients within this  study.
study.
Remaining transvalvular gradients are still a significant problem after ViV procedures. The Global Valve-in-Valve Registry reports high postprocedural gradient in 28.4%, ie, a relatively high rate. The two independent predictors are the severity of bioprosthetic stenosis and the use of an Edwards Sapien valve. Unexpectedly, high postprocedural gradients (>20 mm Hg) were measured in 2 of the 4 present cases despite using the MCV device with its supra-annular function (leaflets open and close in supra-annular position) and slim stent design. However, all patients showed improved clinical symptoms, even those with mean gradients >20 mm Hg, suggesting a clinical benefit of the valve-in-valve therapy. Comparing pre- and postprocedural gradients, we found an overall reduction that might explain symptom relief despite mildly elevated transvalvular gradients. Mildly elevated gradients seem acceptable for valve-in-valve in small bioprostheses, but further long-term follow-up is necessary to evaluate its impact.
Special aspects of CoreValve Evolut. Until recently, only 26 mm, 29 mm, and 31 mm CoreValve prostheses were available and thus were excessively oversized for the Mitroflow 21 with its internal stent diameter of just 17.3 mm. Implantation of 26 mm and 29 mm CoreValves has been described in the literature,7,12 but long-term follow-up data will be mandatory to evaluate the effect of this valve underexpansion with accompanying residual transvalvular gradients. The 23 mm CoreValve Evolut has recently received CE certification and is earmarked for native annular sizes between 18-20 mm. Currently, the CoreValve Evolut is only available within Europe. For our 4 patients, we decided on the 23 mm CoreValve Evolut for ViV implantation into 21 mm Mitroflow prostheses. The in-flow segment of the stent, providing a high radial force, is fitted into the radiopaque sewing ring of the degenerated Mitroflow, aiming to position the CoreValve 4 to 6 mm below the radiopaque marker. The center portion of the CoreValve Evolut is constrained to resist size and shape deformation and due to the valve’s concave design, blood flow to the coronary arteries is secured despite the supra-annular position of the valve leaflets. Therefore, this valve seems very suitable for this type of procedure.
The roles of predilatation and postdilation. The literature on predilatation of failed bioprostheses is controversial and the actual effectiveness and the potential risks of predilatation are not known in great detail.13 However, according to the European and American guidelines, balloon valvuloplasty of stenotic left-sided bioprostheses should be avoided.14,15 In our series, no predilatation of the failed Mitroflow prosthesis was performed to avoid debris embolization, deformation of the stent frame, or stent fracture.
In our case series of 4 patients, no significant aortic regurgitation was observed after implantation. In case of more than moderate regurgitation after the ViV procedure with anatomically correct positioning, we strongly advise against postdilatation, as the in-flow portion of the CoreValve should fit correctly into the radiopaque sewing ring of the Mitroflow valve and the sewing ring should not be dilated, as the inflated balloon would distribute its radial force on the center portion of the Mitroflow, which may then be deformed resulting in subsequent severe problems such as coronary artery occlusion. In our opinion, more than moderate regurgitation of a ViV CoreValve inside a Mitroflow prosthesis can only be explained by leaflet dysfunction or malpositioning and the only acceptable strategy is retrieval of the CoreValve while it is still attached to the delivery catheter or implantation of a second transcatheter valve. It is crucial to rule out paravalvular leakage of the Mitroflow prosthesis as the cause for severe aortic regurgitation before ViV therapy.
Conclusion
Bioprosthetic valve failure can be safely treated by transfemoral TAVI using the 23 mm Medtronic CoreValve Evolut irrespective of the failure mode. Ostial coronary obstruction seems unlikely, but mildly elevated levels of postprocedural gradients are observed without serious clinical impact so far. Further experience for ViV-TAVI in small failed bioprostheses is needed, especially in cases of small degenerated Mitroflow prostheses. Long-term follow-up is required to assess the efficacy of this approach.
References
- Brown JM, O’Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg. 2009;137(1):82-90.
- Glower DD, Landolfo KP, Cheruvu S, et al. Determinants of 15-year outcome with 1,119 standard Carpentier-Edwards porcine valves. Ann Thorac Surg. 1998;66(6 Suppl):S44-S48.
- Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg. 2013;145(1):6-23.
- Grube E, Schuler G, Buellesfeld L, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol. 2007;50(1):69-76.
- Vahanian A, Alfieri O, Al-Attar N, et al; European Association of Cardio-Thoracic Surgery; European Society of Cardiology; European Association of Percutaneous Cardiovascular Interventions. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2008;29(11):1463-1470.
- Ferrari E. Transapical aortic ‘valve-in-valve’ procedure for degenerated stented bioprosthesis. Eur J Cardiothorac Surg. 2012;41(3):485-490.
- Bedogni F, Laudisa ML, Pizzocri S, et al. Transcatheter valve-in-valve implantation using CoreValve Revalving System for failed surgical aortic bioprostheses. JACC Cardiovasc Interv. 2011;4(11):1228-1234.
- Eggebrecht H, Schäfer U, Treede H, et al. Valve-in-valve transcatheter aortic valve implantation for degenerated bioprosthetic heart valves. JACC Cardiovasc Interv. 2011;4(11):1218-1227.
- Azadani AN, Jaussaud N, Matthews PB, Ge L, Chuter TA, Tseng EE. Transcatheter aortic valves inadequately relieve stenosis in small degenerated bioprostheses. Interact Cardiovasc Thorac Surg. 2010;11(1):70-77.
- Gurvitch R, Cheung A, Bedogni F, Webb JG. Coronary obstruction following transcatheter aortic valve-in-valve implantation for failed surgical bioprostheses. Catheter Cardiovasc Interv. 2011;77(3):439-444.
- Dvir D, Webb J, Brecker S, Bleiziffer S, et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the global valve-in-valve registry. Circulation. 2012;126(19):2335-2344.
- Hernández-García JM, Muñoz-García AJ, Alonso-Briales JH, et al. Percutaneous treatment of a dysfunctional aortic bioprosthesis with the CoreValve prosthesis. Rev Esp Cardiol. 2011;64(2):155-158.
- Webb J. Balloon valvuloplasty for stenotic bioprosthetic aortic valves. Catheter Cardiovasc Interv. 2011;77(4):593.
- Vahanian A, Baumgartner H, Bax J, et al; Task force on the management of valvular heart disease of the European Society of Cardiology; ESC Committee for Practice Guidelines (2007). Guidelines on the management of valvular heart disease: the task force on the management of valvular heart disease of the European Society of Cardiology. Eur Heart J. 2007;28(2):230-268.
- Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease). J Am Coll Cardiol. 2006;48(3):e1-e148.
______________________________
From the 1Department of Cardiology and 2Department of Thoracic and Cardiovascular Surgery, Heart and Diabetes Center North Rhine-Westphalia, Ruhr University Bochum, Bad Oeynhausen, Germany.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted October 2, 2013, provisional acceptance given October 25, 2013, final version accepted November 25, 2013.
Address for correspondence: Smita Jategaonkar, MD, Heart and Diabetes Center North Rhine-Westphalia, Department of Cardiology, Georgstr. 11, D-32545 Bad Oeynhausen, Germany. Email: akleemeyer@hdz-nrw.de
















