Total IN.PACT All-Subjects One-Year Analysis and Standard vs Broader Implications
Abstract: Drug-coated balloons (DCBs) have been shown to be superior to percutaneous transluminal angioplasty (PTA) for symptomatic femoropopliteal disease in randomized clinical trials; however, their clinical effectiveness and safety in more complex disease is less defined. The study sought to conduct a patient-level pooled analysis of all prospective randomized and single-arm studies evaluating the safety and efficacy of IN.PACT Admiral DCB (Medtronic) worldwide and in patients with complex disease. Subjects were treated with either IN.PACT Admiral DCB (n = 1837) or PTA (n = 143). The primary endpoint was freedom from clinically driven target-lesion revascularization (CD-TLR) within 12 months. The primary safety composite endpoint was freedom from device- and procedure-related death through 30 days, and freedom from major target-limb amputation, clinically driven target-vessel revascularization, and thrombosis within 12 months. Subsequently, we examined “real-life” complex lesions in a subgroup analysis, with standard use defined as simple, single de novo lesions (n = 712) and broader use defined as bilateral or multiple lesions (n = 1125). DCB when compared with PTA had significantly higher rates of freedom from CD-TLR through 12 months (93.8% vs 80.2%, respectively; P<.001). The DCB group did note higher rates of mortality at 1 year (3.1% DCB vs 0.0% PTA; P=.03). Notably, the broader use group showed superiority over the PTA group for freedom from CD-TLR (91.7% vs 80.2%; P<.001). IN.PACT Admiral DCB showed clinical superiority to PTA in the largest patient-level pooled analysis. Additionally, despite more complex and challenging lesions, DCB was superior to PTA. However, further adequately powered randomized studies are needed to confirm these results.
Key words: drug-coated balloon, percutaneous transluminal angioplasty, peripheral artery disease, target-lesion revascularization
A number of randomized clinical trials (RCTs) have shown the superiority of drug-coated balloons (DCBs) compared with percutaneous transluminal angioplasty (PTA).1-3 However, these trials excluded complex patients with in-stent restenosis or long total occlusions. Prior studies also had small sample sizes, limiting the ability to assess efficacy across broader groups of patients. In the current Total IN.PACT All-Subjects analysis, we conducted the largest patient-level pooled analysis designed to assess clinical efficacy of DCB compared with PTA. More importantly, we examined the clinical efficacy of DCBs on “real-world” patients compared with standard patients enrolled in RCTs and those undergoing PTA.
Methods
Clinical trial registration information is available at www.clinicaltrials.gov (NCT01175850, NCT01566461, NCT01947478, NCT02118532, and NCT01609296).
Study design. The Total IN.PACT All-Subjects study is a pooled analysis that sought to assess the efficacy and safety of IN.PACT Admiral paclitaxel drug-coated balloon (Medtronic) vs PTA across a multicenter, multinational, and heterogeneous population of patients with symptomatic femoropopliteal artery disease. This total pooled analysis is comprised of two RCTs (IN.PACT SFA I and II and IN.PACT Japan) and two prospective single-arm studies (IN.PACT China and IN.PACT Global) whose designs have been previously described (Table 1).4-8
Inclusion criteria were consistent among the IN.PACT SFA trial, IN.PACT Japan trial, and IN.PACT China study. Patients between the ages of 20-85 years with moderate to severe intermittent claudication and/or ischemic rest pain (Rutherford class 2-4) were eligible. Additionally, subjects were included if lesions were de novo or non-stented restenotic, had diameter stenosis 70%-99%, and length 4-20 cm (18 cm maximum for IN.PACT SFA). Chronic total occlusions were allowed if lesion lengths were ≤10 cm long. Severely calcified lesions were excluded. Inclusion criteria were similar, but more permissive for the IN.PACT Global study. Patients 18 years or older with moderate to severe intermittent claudication and/or ischemic rest pain (Rutherford class 2-4) were eligible. Due to protocol violations, one Rutherford class 1 subject and 10 Rutherford class 5 subjects were enrolled and included in the analysis. Subjects were included if lesions were de novo or restenotic (in-stent or non-stented) with lesion length ≥2 cm. Chronic total occlusions were allowed if lesion length was ≥5 cm. Severely calcified lesions were included.
Follow-up was conducted at 30 days, 6 months, and 12 months by the treating physician.
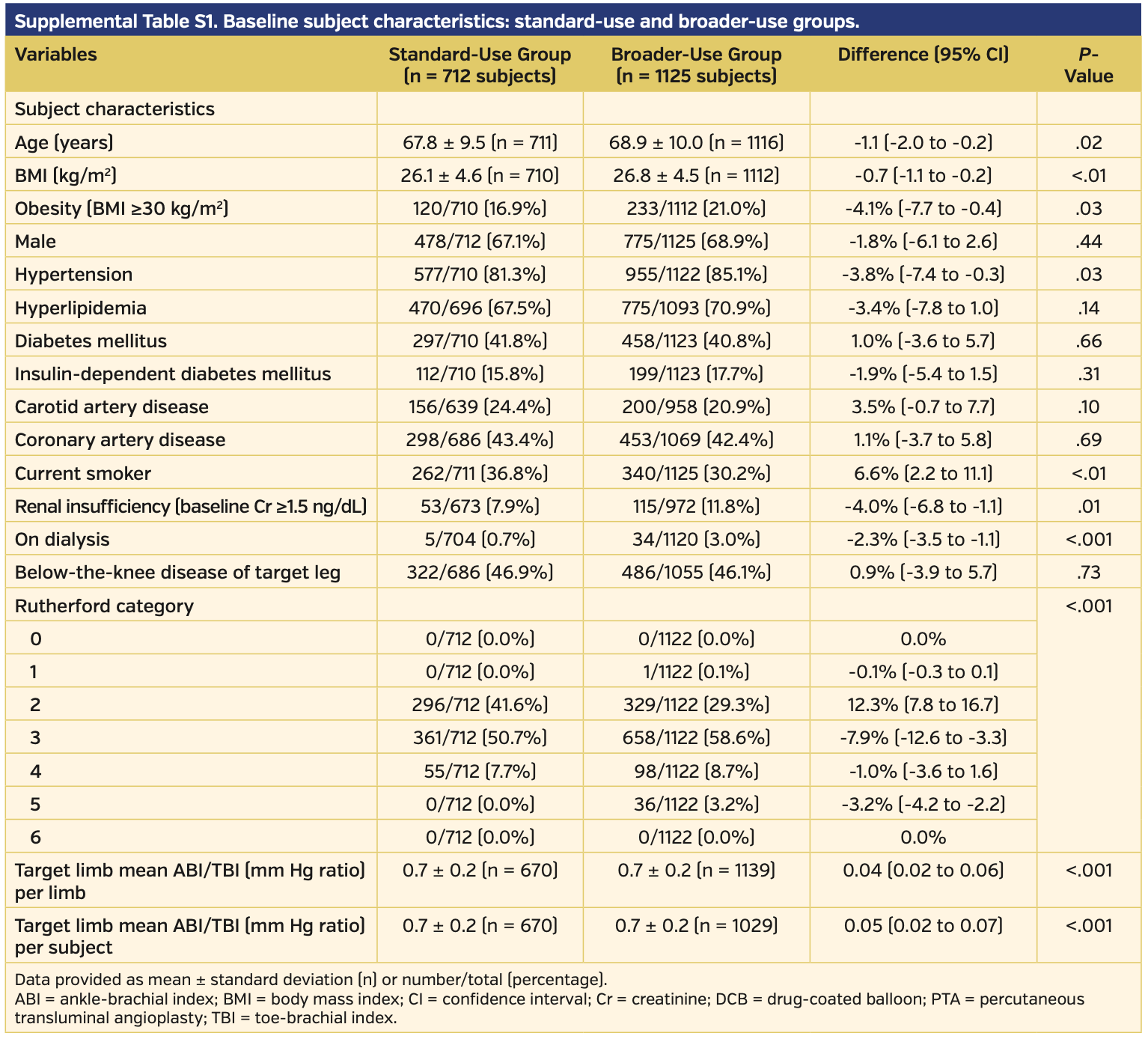
Definitions. The standard-use vs broader-use subgroup analysis comprised subjects from both the two RCTs and the two single-arm, prospective studies. Standard use was defined as single, simple, de novo lesions with lengths ≤20 cm, total occlusions ≤10 cm, and mild to no calcification. Broader use was defined as complex patients and lesions, typically excluded from previous trials and included bilateral, multiple lesions, calcium (moderate to severe), in-stent restenosis, and all subjects who did not meet the standard-use criteria.
The institutional review board of each study center approved the study protocol. Informed consent was obtained from all subjects. The trial was conducted in accordance with the Declaration of Helsinki, good clinical practice guidelines, and applicable laws as specified by all relevant government authorities.
Endpoints. The primary endpoint of this study was freedom from clinically driven target-lesion revascularization (CD-TLR) within 12 months. CD-TLR was defined as any reintervention within the target lesion(s) due to symptoms or drop of ankle-brachial index (ABI) of ≥20% or >0.15 when compared to post-index procedure baseline ABI. The primary safety composite endpoint was freedom from device- and procedure-related death through 30 days, and freedom from major target-limb amputation, clinically driven target-vessel revascularization (TVR), and thrombosis within 12 months. Major adverse events are defined as all-cause mortality, clinically driven TVR, major target-limb amputation, and thrombosis at the target-lesion site. Thrombosis was defined as thrombus formation in the setting of symptoms and confirmed with duplex ultrasonography or angiography. All revascularization and safety events were adjudicated by a clinical events committee (CEC).
Additionally, sustained primary clinical improvement at 12 months was defined as freedom from target-limb amputation, TVR, or an increase in Rutherford classification. Subjects underwent a functional assessment using a Walking Impairment Questionnaire (WIQ), Six Minute Walk test, and a five-dimension (EQ-5D) health status questionnaire at 12 months. All endpoints were adjudicated by an independent CEC.
Procedural outcomes. Device success was defined as successful delivery, inflation, deflation, and retrieval of the intact study balloon device without burst below the rated burst pressure. Procedural success was defined as residual stenosis of ≤50% (non-stented subjects) or ≤30% (stented subjects). Clinical success was defined as procedural success without procedural complications (death, major target-limb amputation, thrombosis of the target lesion, or TVR).
Statistical analysis. Continuous variables for both outcomes and baseline characteristics were described as mean ± standard deviation. Student’s t-test or Wilcoxon rank-sum tests were used to compare continuous variables. For categorical variables, Fisher’s Exact test was used for binary variables. These analyses were used for both the primary and safety endpoints. Kaplan-Meier method was used to report time-to-event data for freedom from CD-TLR over the 12-month follow-up period. A log-rank test was used to compare the survival curves between treatment groups in both the pooled analysis as well as the subgroup analysis. Statistical significance was defined as P<.05. All statistical analyses were performed using the intent-to-treat principle by an independent party (The Baim Institute for Clinical Research, formerly HCRI; Boston, Massachusetts) in order to mitigate analytical bias.
Results
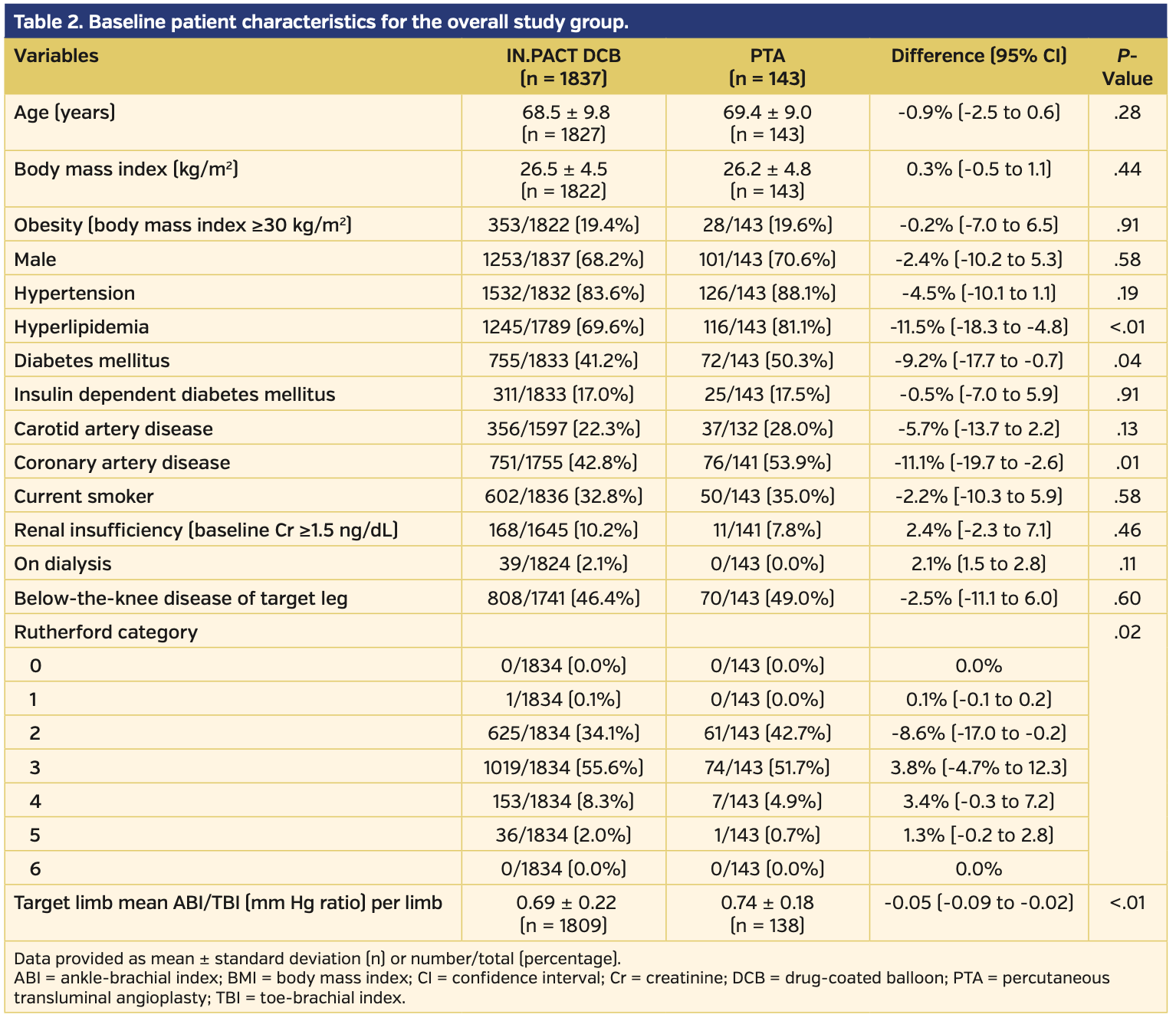
Overall cohort. The total pooled analysis included 1837 subjects treated with DCB and 143 subjects who underwent PTA for symptomatic femoropopliteal disease. Baseline characteristics are reported in Table 1. The DCB group had lower rates of hyperlipidemia, diabetes, and history of coronary disease when compared with the PTA group. However, the DCB subjects had more advanced symptoms and disease, as defined by higher Rutherford classification (P=.02) and lower mean ABI/toe-brachial index ratio compared with those treated with PTA (P<.01) (Table 2). All other baseline characteristics were similar between the DCB and PTA cohorts.
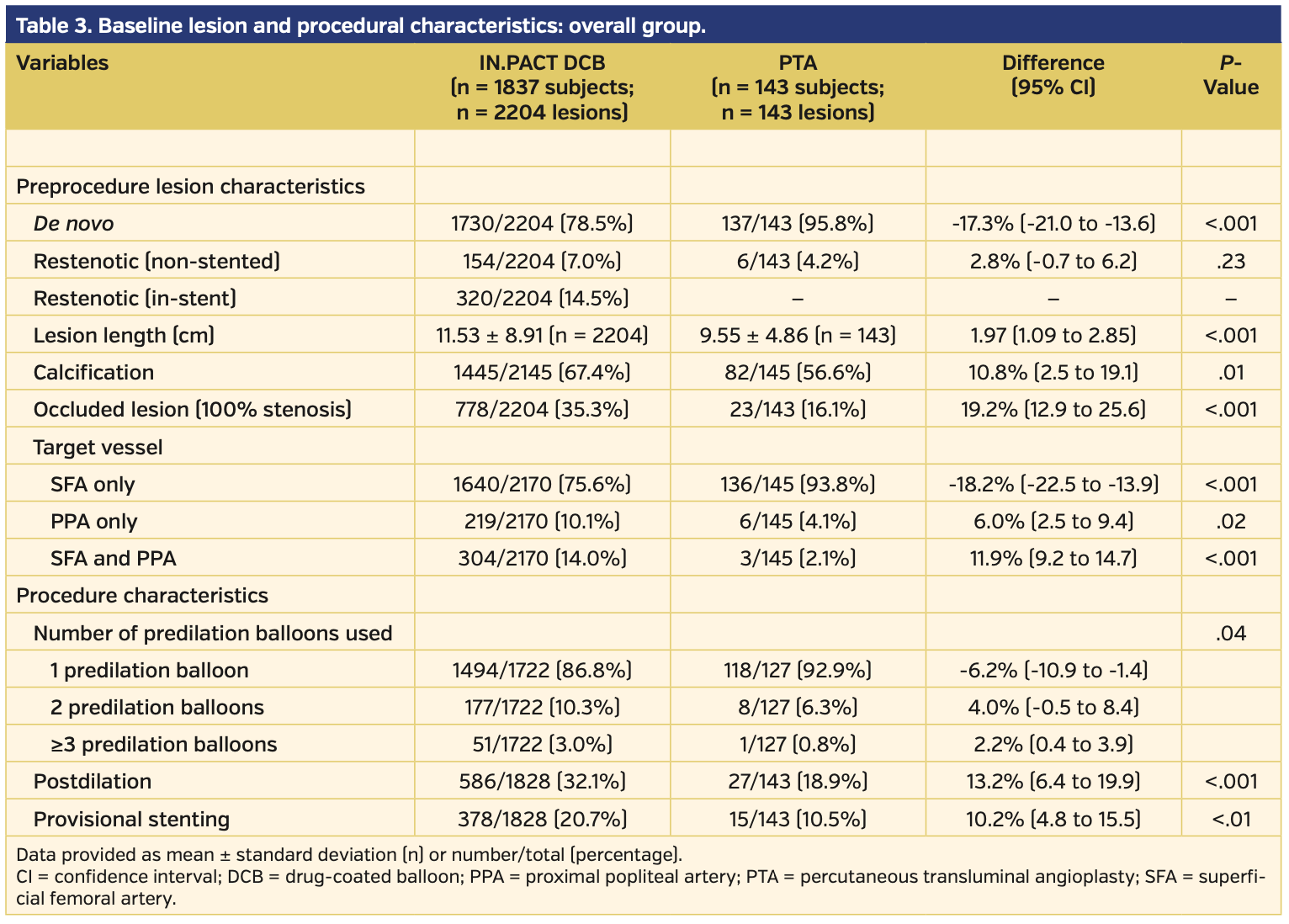
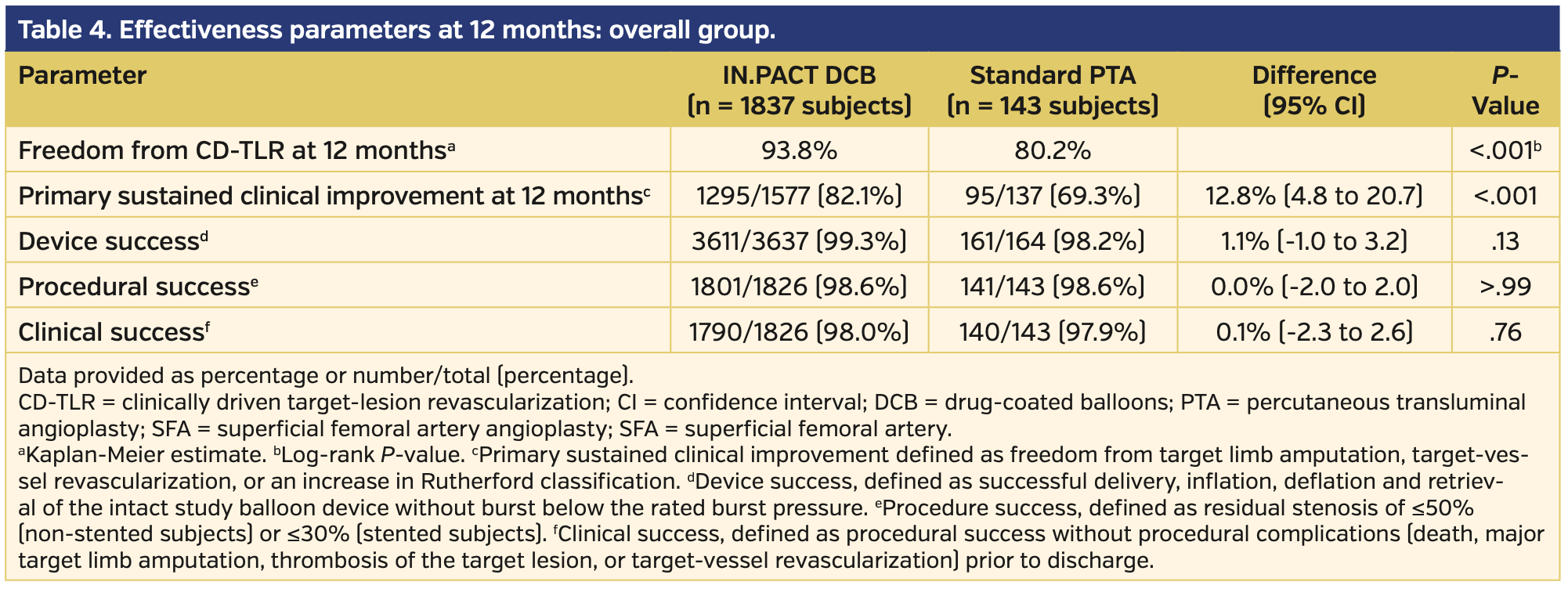
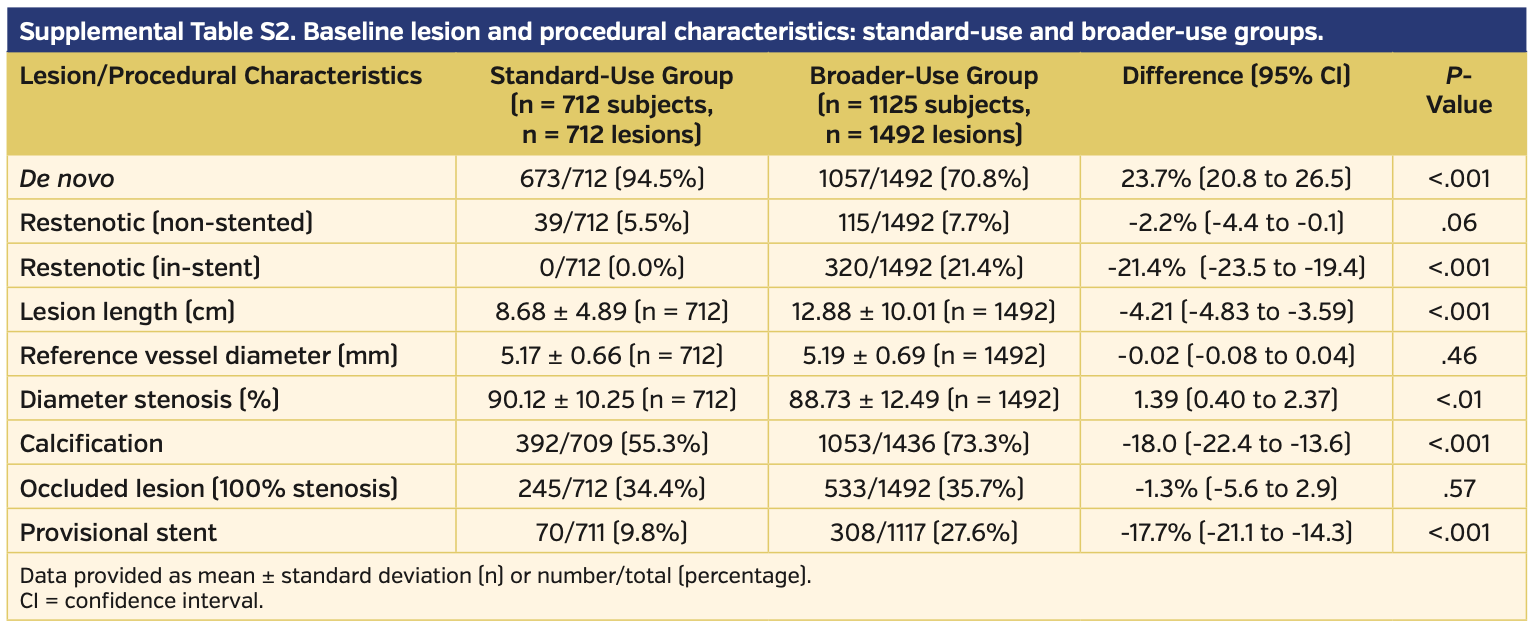
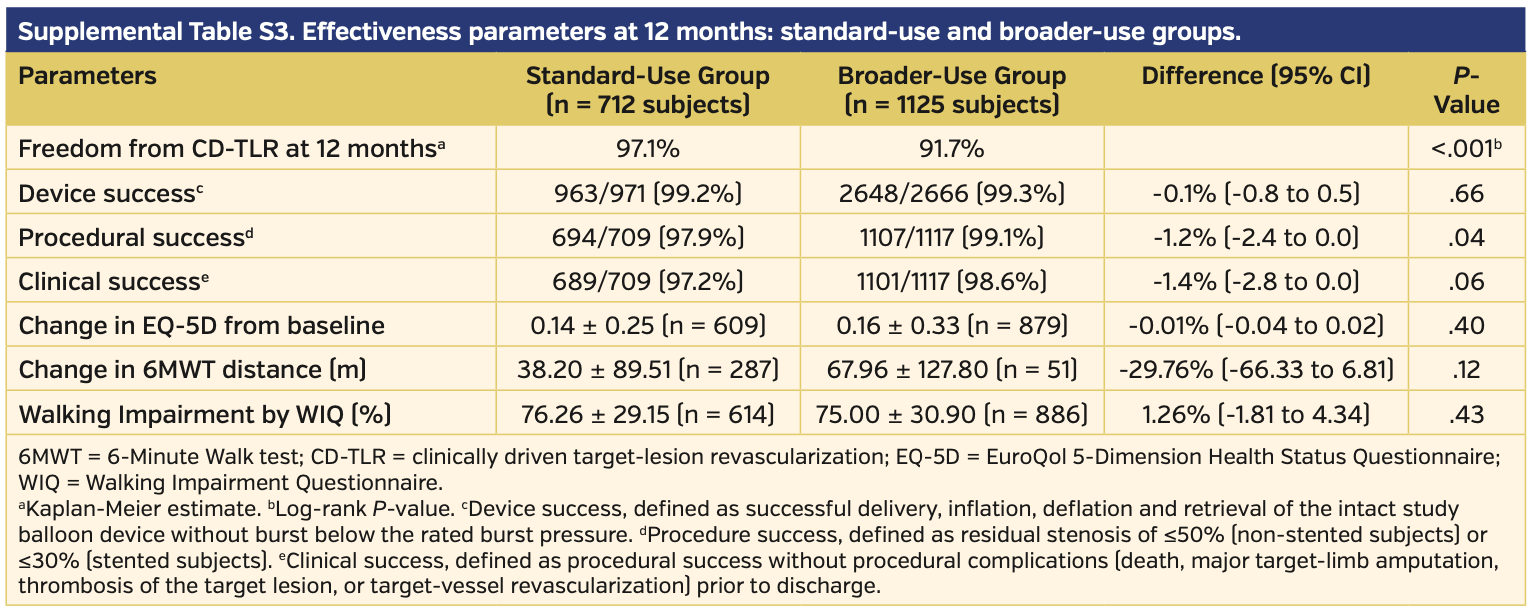
Angiographically, subjects treated with DCB had longer lesion lengths, higher prevalence of chronically occluded lesions, and more calcification vs those treated with PTA (Table 3). There were higher rates of provisional stents used during initial intervention in the DCB vs PTA subject population (20.7% vs 10.5%, respectively; P<.01). Clinical success prior to discharge was similar between the DCB and PTA subject groups (98.0% vs 97.9%, respectively; P=.76) (Table 4).
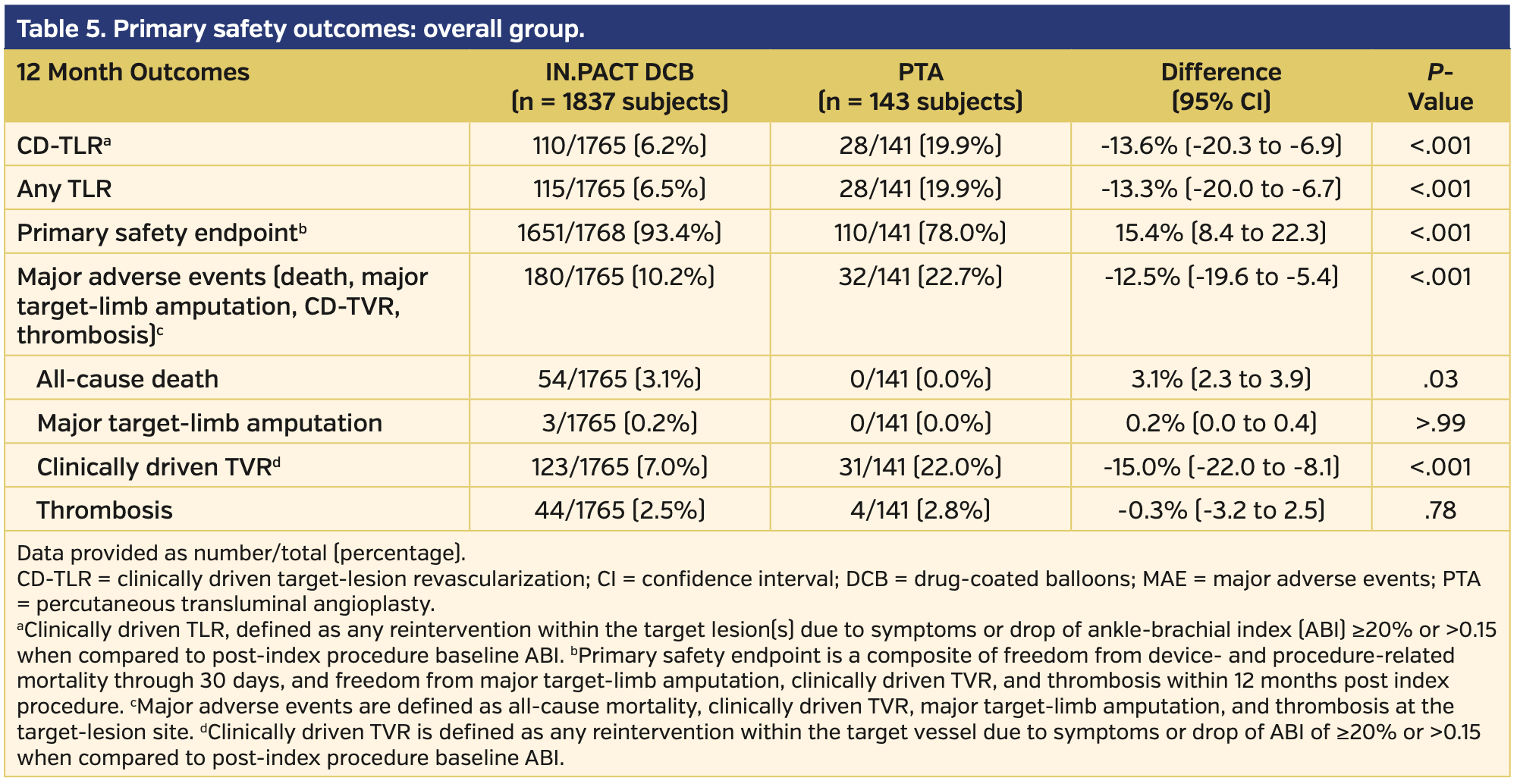
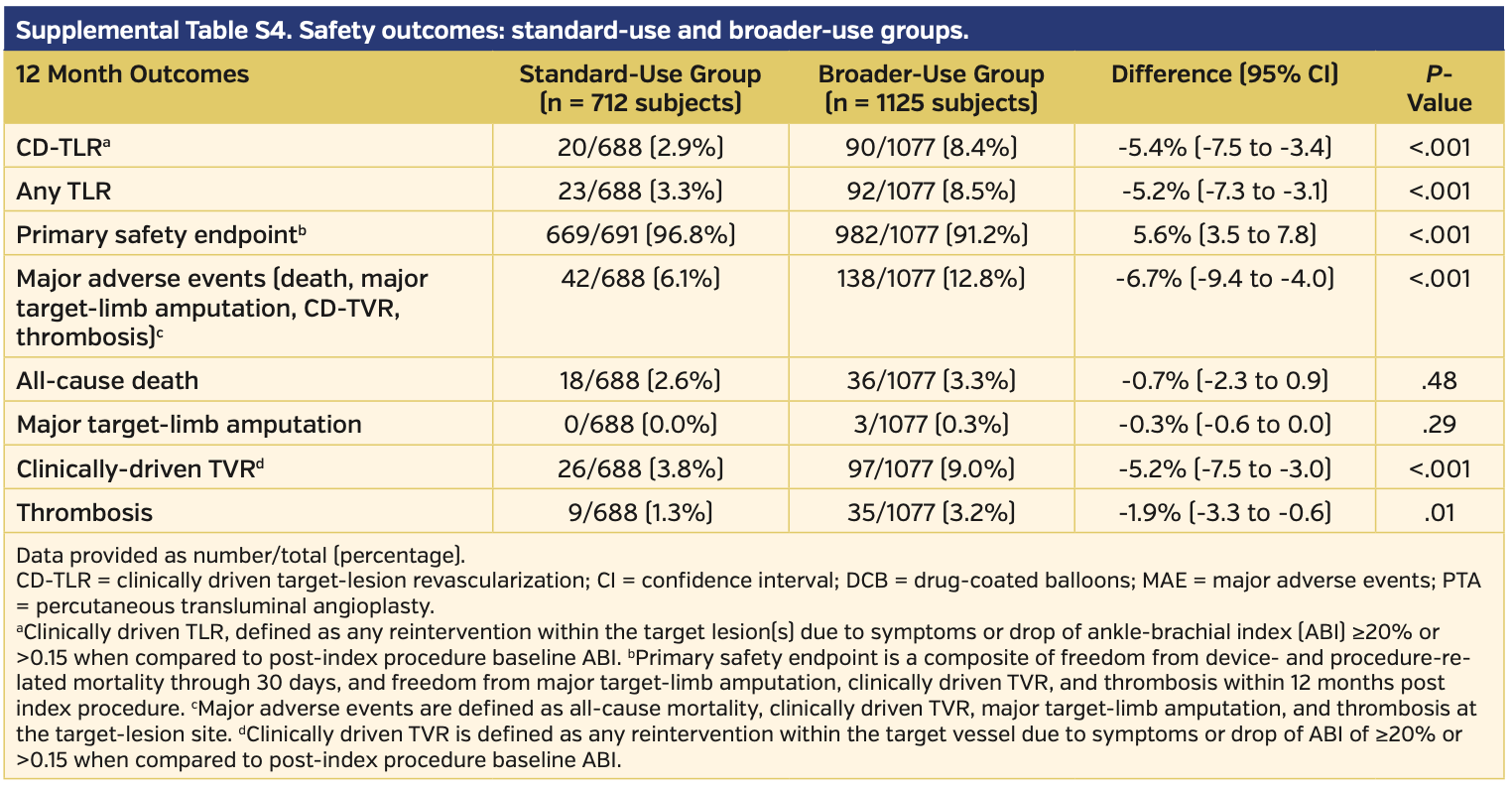
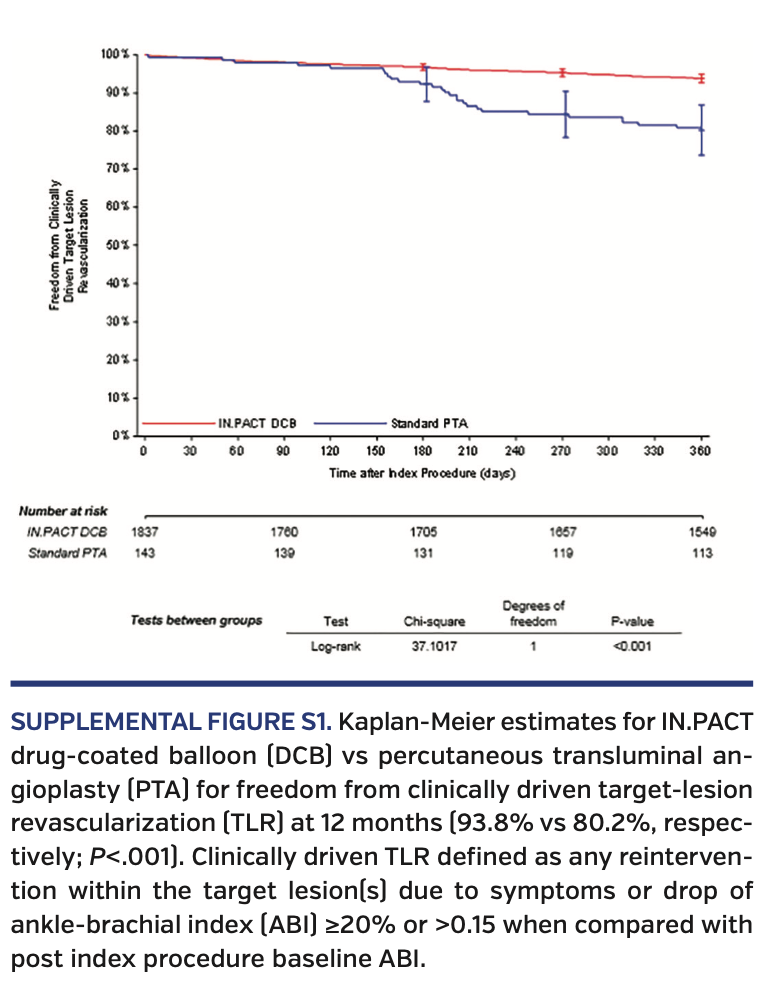
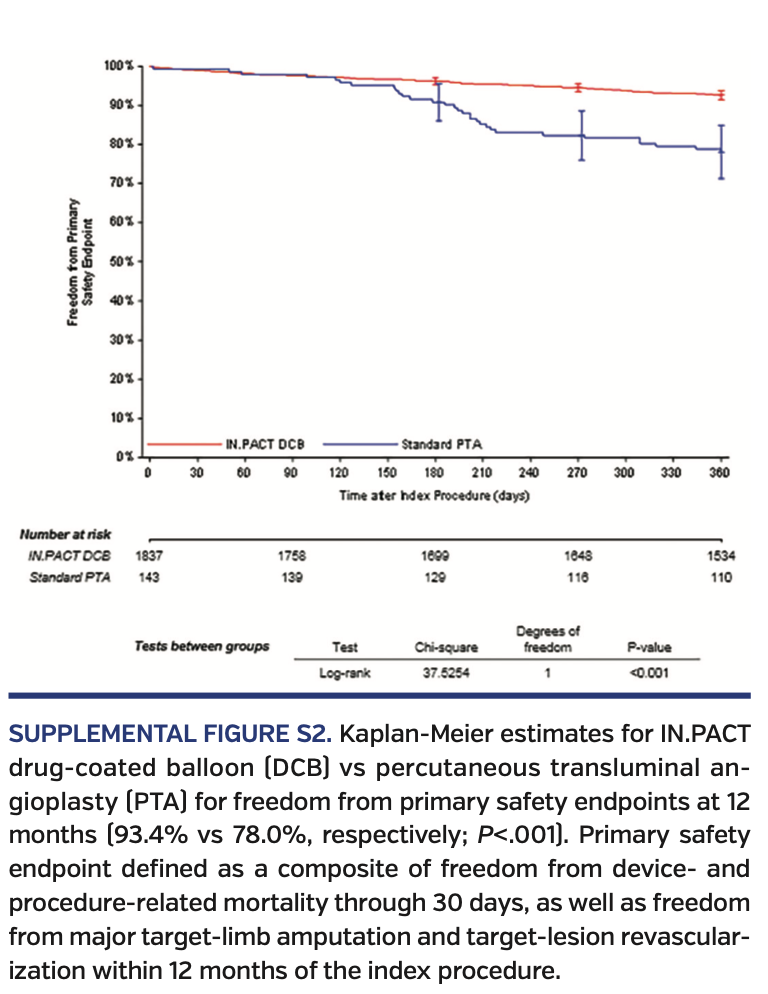
Primary endpoints. At 12 months, DCB-treated subjects had higher rates of freedom from CD-TLR vs subjects undergoing PTA (93.8% vs 80.2%, respectively; P<.001) (Supplemental Figure S1; supplemental materials available at www.invasivecardiology.com). The primary composite safety endpoint was superior for DCB compared with PTA. At 360 days, 93.4% of DCB subjects met the primary safety endpoint vs 78.0% for PTA subjects (P<.001) (Supplemental Figure S2). The remaining primary safety outcome details are available in Table 5. There was an increase in all-cause death in the DCB cohort comparatively (3.1% vs 0.0%; P=.03). However, of note, the cumulative 360-day major adverse events, which included death, major target-limb amputation, clinically driven TVR, and thrombosis, were significantly higher in the PTA group vs the DCB group (22.7% vs 10.2%, respectively; P<.001).
Secondary endpoints. Using the EQ-5D index as reference, there was a significant quality of life improvement in the DCB group vs the PTA group, as defined as change from baseline at 12 months (0.15 vs 0.08, respectively; P<.001). However, there was no statistical difference in patients’ symptoms with ambulating as assessed by a Six-Minute Walk test (measured in meters) and WIQ score (42.69 ± 96.63 vs 46.25 ± 86.41 [P=.78] and 75.52 ± 30.19 vs 77.24 ± 28.01 [P=.52], respectively).
Subgroup analysis for standard vs broader use. Of the total pooled analysis, 712 subjects met the standard-use definition and 1125 patients met the broader-use definition. Baseline characteristics demonstrated higher rates of comorbidities in the broader-use group, with higher age and higher rates of obesity, hypertension, and renal insufficiency as well as more advanced peripheral disease compared with the standard-use group (Supplemental Table S1). Angiographically, the broader-use group had significantly more-challenging lesions and a higher rate of provisional stent use (Supplemental Table S2). Clinical success was similar between the two groups, and in fact favored the broader-use group (97.2% in the standard-use group vs 98.6% in the broader-use group; P=.06) (Supplemental Table S3). There was a higher rate of freedom from CD-TLR in the standard-use group vs the broader-use group (97.1% vs 91.7%, respectively; P<.001); however, the broader-use group still outperformed PTA (91.7% vs 80.2%, respectively; P<.001) (Supplemental Figure S3). There was no difference between quality of life or walking functional status when comparing EQ-5D, Six-Minute Walk test, and WIQ between the standard-use and broader-use groups (Supplemental Table S3). The primary safety endpoint was met by the standard-use group more often than in the broader-use group (96.8% vs 91.2%, respectively; P<.001) (Supplemental Table S4).
Discussion
In this largest patient-level analysis of DCB effectiveness, it was shown that the IN.PACT Admiral DCB is superior to PTA for symptomatic femoropopliteal artery disease. More importantly, the IN.PACT Admiral DCB showed superior efficacy and safety compared with PTA regardless of standard or broader use. These findings confirm the safety and efficacy of DCB compared with PTA for simple and complex lesions.
Despite more severe Rutherford classification scores and lower mean ABIs (indicative of overall worse disease), the DCB subjects had higher rates of freedom from CD-TLR compared with PTA at 12 months. Of note, functional outcomes were comparable between the two groups, while the PTA group required more interventions to achieve the same values. Due to the large subject population, the Total IN.PACT All-Subjects analysis reiterates the effectiveness of treating femoropopliteal artery disease with DCBs on a global scale. Even with longer lesion lengths and higher proportion of occluded lesions, the IN.PACT Admiral DCB still outperformed PTA. Of note, subjects treated with DCB did have higher rates of bailout stenting than PTA subjects, but this is largely reflective of the longer, more complex lesions in a sicker (as reflected by lower mean ABIs) and more symptomatic patient population. Furthermore, the PTA group represents patients only enrolled in randomized investigational drug evaluation (IDE) clinical trials with strict inclusion and exclusion criteria, while 76.5% of the patients in the DCB group are from the IN.PACT Global registry, which represents real-world lesions with no angiographic exclusions.
The standard-use group did have higher rates of freedom from CD-TLR compared with the broader-use group, which speaks to the severity of disease in the latter group. However, the broader usage still outperformed the PTA group. This not only highlights the complexity of lesions typically seen in daily practice and how DCBs serve as an important means for SFA treatment, but also reiterates the clinical superiority of DCB over PTA for even complex lesions. In an effort to maximize a “leave nothing behind” strategy, DCBs offer an alternative and better approach for symptomatic femoropopliteal disease.
Our analysis also highlights the safety of DCB over PTA. The primary safety endpoint was met at higher rates in the DCB group vs the PTA group (93.4% vs 78.0%, respectively; P<.001). In a recent meta-analysis comprising 28 randomized control trials, Katsanos et al raised concerns of increased rates of mortality associated with DCBs.9 While the current analysis does not directly address this concern, two recent publications from the same data have shown no statistically significant association between the IN.PACT Admiral DCB and mortality.10-12 Collectively, concerns raised by Katsanos et al have also been addressed by other analyses that have also failed to show an association between risk of mortality and use of paclitaxel devices.13,14 Analyses have also shown lack of an association between mortality risk and paclitaxel dose.
Study limitations. The current study has a number of limitations. First, it has a short follow-up of 1 year and longer-term follow-up will be useful to confirm persistent efficacy and safety. Second, patients in the PTA group were mainly enrolled in the IDE trials. Therefore, lesions were simpler compared with the DCB cohort. However, this has most likely attenuated the superiority of DCB over PTA. Third, data on patency were not available for all patients; however, a previous analysis on a smaller cohort with duplex ultrasound core-lab adjudicated data from the Total IN.PACT dataset has shown superior patency with DCB compared with PTA. Fourth, important information regarding medical therapy is missing. Given the recent concerns about mortality, we hope that future trials capture and enforce more aggressive medical therapy and risk-factor modification in patients with symptomatic femoropopliteal disease. Finally, further adequately powered randomized studies are needed to confirm these results.
Conclusion
This study demonstrated clinical superiority of DCB over PTA in the largest multinational, multicenter, randomized analysis to date. Moreover, this study highlighted the safety and efficacy of DCB for real-world lesions, reinforcing the use of DCBs for symptomatic femoropopliteal disease even in complex patients. DCBs should be considered safe and effective for patients with symptomatic femoropopliteal disease with standard lesions, but also for more complex, diseased lesions in an effort to “leave nothing behind.”
From the 1Heart & Vascular Institute, University Hospitals Cleveland Medical Center and Case Western Reserve University School of Medicine, Cleveland, Ohio; 2Harvard Medical School, Boston, Massachusetts; 3Universitäts-Herzzentrum Freiburg–Bad Krozingen, Bad Krozingen, Germany; and 4Hawaii Permanente Medical Group, Kaiser Foundation Hospital, Honolulu, Hawaii.
This work was presented at the Transcatheter Cardiovascular Therapeutics 2018 Late Breaking Clinical Trial session.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Jaff reports non-compensated consultant work for Abbott Vascular, Boston Scientific, Cordis Corporation, and Medtronic; consultant fees from AOPA, Micell, Primacea, Silk Road Medical, Vactronix, Venarum, and Volcano/Philips; equity in Embolitech, Gemini, Janacare, MC10, Northwind Medical, PQ Bypass, Primacea, Sano V, and Vascular Therapies; board member of Greenway Health. Dr Zeller declares honoraria from Abbott Vascular, Veryan, Biotronik, Boston Scientific, Cook Medical, Gore and Associates, Medtronic, Philips-Spectranetics, TriReme, and Shockwave; consultant income from Boston Scientific, Cook Medical, Gore and Associates, Medtronic, Spectranetics, Veryan, Intact Vascular, B. Braun, Shockwave, and Bayer; research, clinical trial, or drug study support from 480 Biomedical, Bard Peripheral Vascular, Veryan, Biotronik, Cook Medical, Gore and Associates, Medtronic, Philips, Terumo, TriReme, Shockwave, Med Alliance, Intact Vascular, and B. Braun; common stock in Veryan and QT Medical. Dr Schneider reports a modest royalty from Cook Medical; member of the Scientific Advisory Board at Medtronic, Abbott, CSI, and Boston Scientific; Chief Medical Officer for Intact Vascular and Cagent; teaching honorarium from Medtronic. Dr Shishehbor reports consultant income from Abbott Vascular, Medtronic, Boston Scientific, Philips, and Terumo. The remaining authors report no conflicts of interest regarding the content herein.
Manuscript submitted April 8, 2020, provisional acceptance given April 13, 2020, final version accepted May 12, 2020.
Address for correspondence: Mehdi H. Shishehbor, DO, MPH, PhD, Professor of Medicine, Case Western Reserve University School of Medicine, University Hospitals, 11100 Euclid Avenue, Lakeside 3rd Floor, Cleveland, OH 44106. Email: Mehdi.Shishehbor@UHhospitals.org
- Krishnan P, Faries P, Niazi K, et al. Stellarex drug-coated balloon for treatment of femoropopliteal disease: twelve-month outcomes from the randomized ILLUMENATE pivotal and pharmacokinetic studies. Circulation. 2017;136:1102-1113.
- Rosenfield K, Jaff MR, White CJ, et al. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med. 2015;373:145-153.
- Scheinert D, Duda S, Zeller T, et al. The LEVANT I (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first-in-human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplasty. JACC Cardiovasc Interv. 2014;7:10-19.
- Laird JR, Schneider PA, Tepe G, et al. Durability of treatment effect using a drug-coated balloon for femoropopliteal lesions: 24-month results of IN.PACT SFA. J Am Coll Cardiol. 2015;66:2329-2338.
- Iida O, Soga Y, Urasawa K, et. al. Drug-coated balloon vs standard percutaneous transluminal angioplasty for the treatment of atherosclerotic lesions in the superficial femoral and proximal popliteal arteries: one-year results of the MDT-2113 SFA Japan randomized trial. J Endovasc Ther. 2018;25:109-117.
- Tepe G, Laird J, Schneider P, et al. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131:495-502.
- Ansel GM, Brodmann M, Keirse K, et al. Drug-coated balloon treatment of femoropopliteal lesions typically excluded from clinical trials: 12-month findings from the IN.PACT Global Study. J Endovasc Ther. 2018;25:673-682.
- Chen Z, Guo W, Jiang W, et al. IN.PACT SFA Clinical Study using the IN.PACT Admiral drug-coated balloon in a Chinese patient population. J Endovasc Ther. 2019;26:471-478.
- Katsanos K, Spiliopoulos S, Kitrou P, et al. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2018;7:e011245.
- Schneider PA, Laird JR, Doros G, et al. Mortality not correlated with paclitaxel exposure: an independent patient-level meta-analysis of a drug-coated balloon. J Am Coll Cardiol. 2019;73:2550-2563.
- Laird JA, Schneider PA, Jaff MR, et al. Long-term clinical effectiveness of a drug-coated balloon for the treatment of femoropopliteal lesions. Circ Cardiovasc Interv. 2019;12:e007702.
- Banerjee S, Shishehbor MH. Aere perennius. Circ Cardiovasc Interv. 2019;12:e008088.
- Secemsky EA, Kundi H, Weinberg I, et al. Association of survival with femoropopliteal artery revascularization with drug-coated devices. JAMA Cardiol. 2019;4:332-340.
- Shishehbor MH, Secemsky EA, Varcoe RL. Is there a real association between paclitaxel devices and mortality? Time to pause and re-evaluate what we know about this statistical finding. J Am Heart Assoc. 2019;8:e012524.