Successful Treatment of SVC Syndrome Using Isolated Pharmacomechanical Thrombolysis
ABSTRACT: We report 2 cases of superior vena cava (SVC) syndrome treated using isolated pharmacomechanical thrombolysis with angioplasty alone. We also propose a new staged treatment strategy to optimally manage such patients, taking into consideration both cost-effectiveness and the ultimate prognosis.
J INVASIVE CARDIOL 2012;24(3):E50-E53
_________________________________________________
Superior vena cava (SVC) syndrome is a rare condition that results from obstruction of the blood flow through the SVC. Multiple etiologies have been described with malignancy being the most common.1 Obstruction can be secondary to external compression by an invasive malignant tumor, lymph node enlargement, fibrosing mediastinitis, other mediastinal structures or thrombosis within the SVC. Both external compression and thrombosis can coexist.1 Despite the fact that endovascular stents are currently considered the standard of treatment for SVC syndrome,2,3 we report here 2 cases of SVC syndrome treated using only isolated pharmacomechanical thrombolysis (IPMT) and balloon angioplasty with excellent short-term results.
 Case Report 1. A 63-year-old female patient with a past medical history significant for bilateral breast cancer status post bilateral mastectomies, chemotherapy, and radiation therapy presented with a 6-week history of slowly increasing swelling in her upper arms and neck. Her symptoms became suddenly worse 6 days prior to her ER visit and were associated with headaches and orthopnea. Prior to her admission, a chest CT scan with contrast was performed at an outside institution and was inconclusive. Given her clinical
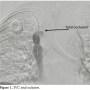
Case Report 1. A 63-year-old female patient with a past medical history significant for bilateral breast cancer status post bilateral mastectomies, chemotherapy, and radiation therapy presented with a 6-week history of slowly increasing swelling in her upper arms and neck. Her symptoms became suddenly worse 6 days prior to her ER visit and were associated with headaches and orthopnea. Prior to her admission, a chest CT scan with contrast was performed at an outside institution and was inconclusive. Given her clinical 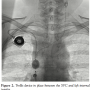 presentation and the fact the she still had a venous port in her right subclavian vein, she was brought straight to the catheterization lab. Following informed consent and moderate sedation, an 8 Fr Pinnacle sheath (Terumo) was placed through the right femoral vein. A venogram of the SVC showed 100% occlusion (Figure 1). After infusion of 4000 international units of IV heparin, a 70 cm 8 Fr cook sheath (Cook Medical) was introduced. Using a Berenstein catheter (AngioDynamics) and a Glidewire
presentation and the fact the she still had a venous port in her right subclavian vein, she was brought straight to the catheterization lab. Following informed consent and moderate sedation, an 8 Fr Pinnacle sheath (Terumo) was placed through the right femoral vein. A venogram of the SVC showed 100% occlusion (Figure 1). After infusion of 4000 international units of IV heparin, a 70 cm 8 Fr cook sheath (Cook Medical) was introduced. Using a Berenstein catheter (AngioDynamics) and a Glidewire  (Terumo), the SVC occlusion was crossed and a venogram of the left internal jugular vein (LIJ) revealed a 100% stenosis of the distal LIJ and left subclavian system. An 8 Fr Trellis was then placed (Figure 2), isolating a 15 cm treatment zone between the SVC and the LIJ. We delivered 10 mg of t-PA over 12 minutes with complete removal of the thrombus. Another venogram of the SVC showed a right innominate vein thrombus. Using a Berenstein catheter (AngioDynamics), we then crossed with an exchange-length Wholey wire (Mallinckrodt) to the right internal jugular vein (RIJ). An 8 Fr Trellis with a 15 cm treatment zone was advanced into the right subclavian vein (RSV) and 10 mg of t-PA were delivered between the SVC and the RSV over a 10-minute period. Following thrombus aspiration, scar around the port just distal to the SVC was noted and was dilated with 10 mm x 40 mm and 12 mm x 40 mm Agiltrac balloons (Abbott Vascular), with excellent results (Figure 3). Patient’s symptoms improved substantially during her hospital stay and she remained asymptomatic at her 6 months post-procedural follow-up visit.
(Terumo), the SVC occlusion was crossed and a venogram of the left internal jugular vein (LIJ) revealed a 100% stenosis of the distal LIJ and left subclavian system. An 8 Fr Trellis was then placed (Figure 2), isolating a 15 cm treatment zone between the SVC and the LIJ. We delivered 10 mg of t-PA over 12 minutes with complete removal of the thrombus. Another venogram of the SVC showed a right innominate vein thrombus. Using a Berenstein catheter (AngioDynamics), we then crossed with an exchange-length Wholey wire (Mallinckrodt) to the right internal jugular vein (RIJ). An 8 Fr Trellis with a 15 cm treatment zone was advanced into the right subclavian vein (RSV) and 10 mg of t-PA were delivered between the SVC and the RSV over a 10-minute period. Following thrombus aspiration, scar around the port just distal to the SVC was noted and was dilated with 10 mm x 40 mm and 12 mm x 40 mm Agiltrac balloons (Abbott Vascular), with excellent results (Figure 3). Patient’s symptoms improved substantially during her hospital stay and she remained asymptomatic at her 6 months post-procedural follow-up visit.
 Case Report 2. A 45-year-old woman with a history of right-sided breast cancer diagnosed 1 year prior to her admission presented to an outside hospital with a 6-week history of slowly progressing neck, facial, breast swelling, and shortness of breath. A CT scan of the chest showed near complete occlusion of the superior vena cava. Patient was transferred to our center for further management. She had received multiple upper extremity ports and catheters over the last year and had a left subclavian line still in place upon presentation.
Case Report 2. A 45-year-old woman with a history of right-sided breast cancer diagnosed 1 year prior to her admission presented to an outside hospital with a 6-week history of slowly progressing neck, facial, breast swelling, and shortness of breath. A CT scan of the chest showed near complete occlusion of the superior vena cava. Patient was transferred to our center for further management. She had received multiple upper extremity ports and catheters over the last year and had a left subclavian line still in place upon presentation. 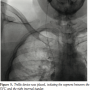 A venogram was obtained using her left subclavian port. The SVC, RIJ, and right brachiocephalic vein were occluded (Figure 4). The right internal mammary vein was large and provided many venous collaterals. We then accessed the right femoral vein, introduced a 10 cm 8 Fr Pinnacle sheath (Terumo) and crossed the obstruction using a 0.035 Glidewire (Terumo) and a 5 Fr angled-tip Berenstein catheter (AngioDynamics). After confirming we were in the true lumen of the RIJ, we exchanged for a Rosen wire
A venogram was obtained using her left subclavian port. The SVC, RIJ, and right brachiocephalic vein were occluded (Figure 4). The right internal mammary vein was large and provided many venous collaterals. We then accessed the right femoral vein, introduced a 10 cm 8 Fr Pinnacle sheath (Terumo) and crossed the obstruction using a 0.035 Glidewire (Terumo) and a 5 Fr angled-tip Berenstein catheter (AngioDynamics). After confirming we were in the true lumen of the RIJ, we exchanged for a Rosen wire 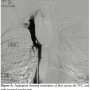 (Infiniti Medical) and introduced an 8 Fr Trellis device with a 15 cm treatment zone. After isolating the segment between the RIJ and the SVC/right atrial junction (Figure 5), we infused 11 mg of t-PA over 12 minutes. Following restoration of flow, a severe stricture was seen in the SVC and the brachiocephalic vein. We then dilated the brachiocephalic vein using an 8 mm x 60 mm Agiltrac balloon (Abbott) at 6 and 12 atm, and dilated the SVC using a 12 mm x 40 mm Foxcross balloon (Abbott) at 8 atm in graded increases of 4 atm. We checked for a pressure gradient between the RIJ and the right atrium using a 6 Fr multipurpose catheter (Boston Scientific), a 0.018 wire, and a Tuohy system (Boston Scientific). The maximal pressure difference was less than 5 mmHg. A venogram was performed and showed good flow restoration across the brachiocephalic and SVC (Figure 6). Patient felt a decrease in her neck swelling and shortness of breath while on the table within a few minutes following the end of the procedure. She was still asymptomatic and feeling great at 3 months follow-up and was later seen by her primary oncologist for continued work-up for cancer.
(Infiniti Medical) and introduced an 8 Fr Trellis device with a 15 cm treatment zone. After isolating the segment between the RIJ and the SVC/right atrial junction (Figure 5), we infused 11 mg of t-PA over 12 minutes. Following restoration of flow, a severe stricture was seen in the SVC and the brachiocephalic vein. We then dilated the brachiocephalic vein using an 8 mm x 60 mm Agiltrac balloon (Abbott) at 6 and 12 atm, and dilated the SVC using a 12 mm x 40 mm Foxcross balloon (Abbott) at 8 atm in graded increases of 4 atm. We checked for a pressure gradient between the RIJ and the right atrium using a 6 Fr multipurpose catheter (Boston Scientific), a 0.018 wire, and a Tuohy system (Boston Scientific). The maximal pressure difference was less than 5 mmHg. A venogram was performed and showed good flow restoration across the brachiocephalic and SVC (Figure 6). Patient felt a decrease in her neck swelling and shortness of breath while on the table within a few minutes following the end of the procedure. She was still asymptomatic and feeling great at 3 months follow-up and was later seen by her primary oncologist for continued work-up for cancer.
 Discussion. Following its first description by William Hunter in 1757,4 SVC syndrome had multiple varying etiologies over the years. In the pre-antibiotic era, SVC syndrome was frequently due to syphilitic thoracic aortic aneurysms, tuberculosis, fibrosing mediastinitis, and other complications of untreated infections.5 In the 1980s, malignancy became the most common cause of SVC obstruction accounting for more than 90% of the cases with bronchogenic carcinoma being the most common cause.6 Thrombosis of the SVC became more frequent following the increase in the use of indwelling catheters7 with pulmonary emboli being a very uncommon manifestation.8
Discussion. Following its first description by William Hunter in 1757,4 SVC syndrome had multiple varying etiologies over the years. In the pre-antibiotic era, SVC syndrome was frequently due to syphilitic thoracic aortic aneurysms, tuberculosis, fibrosing mediastinitis, and other complications of untreated infections.5 In the 1980s, malignancy became the most common cause of SVC obstruction accounting for more than 90% of the cases with bronchogenic carcinoma being the most common cause.6 Thrombosis of the SVC became more frequent following the increase in the use of indwelling catheters7 with pulmonary emboli being a very uncommon manifestation.8
Symptoms of SVC syndrome include dyspnea, facial swelling, head fullness, arm swelling, cough, chest pain,9 and jugular vein distention with absence of venous waveforms. If left untreated, SVC obstruction can cause increased intracerebral pressure, resulting in severe headaches, confusion, and possibly coma. The latter occurs very rarely because venous collaterals develop over time, decreasing the pressure gradient across the obstruction.
Given that many patients suffering from SVC syndrome have stage IV cancer, symptomatic relief of the obstruction is much more important than long-term patency rates.10 Evidence-based guidelines for management of SVC syndrome are currently lacking, but the National Comprehensive Cancer Network (NCCN) and the American College of Chest Physicians (ACCP) made general recommendations supporting radiotherapy and the use of endovascular stents,11,12 when appropriate. Several treatment options have been reported so far with varying degrees of success. When patients’ symptoms are mild, external compression of the SVC can be relieved by conservative measures such as head elevation, glucocorticoids, and diuretics.2 If symptoms are severe enough, treatment of the underlying cause becomes an appealing strategy. However, that is not always devoid of consequences. Radiation therapy, even though very effective in some cases, could mask tumor identification in almost half of the patients.13
Given that the average survival of patients with malignancy complicated by SVC syndrome is 6 months,10 endovascular stents have become the treatment of choice for acute symptom relief of such patients.2 It is worth noting, though, that both benign and malignant causes of SVC syndrome have been successfully treated with percutaneous stent placement.3 Success rates range between 95% and 100% and 1 year primary patency rates range between 64% and 76%.10,14-17 In a large systematic review of patients with lung cancer and SVC syndrome, 95% of 159 patients who underwent endovascular stenting had relief of symptoms, and the incidence of re-occlusion, whether due to thrombosis or tumor in-growth, was only 11%, as compared to 17% to 19% with radiation and/or chemotherapy. When stent placement is difficult or dangerous due to the presence of a large amount of thrombus, thrombectomy or thrombolysis should be attempted prior to stent deployment. Even though the latter strategy has been reported previously with excellent results,14,18 a few issues need to be discussed. Catheter-directed thrombolysis is usually time consuming (12 to 48 H) and when followed by stent placement, it has the potential for doubling the patient’s procedural risk. In addition, the incidence of hematoma, gastrointestinal bleeding, epistaxis, hemoptysis, and stent insertion morbidity rises significantly when thrombolysis precedes stent deployment,1 making this strategy used less often.
On the other hand, stent placement for treatment of SVC syndrome is not devoid of any complications. Infections, pulmonary embolus, stent migration, hematoma at the insertion site, bleeding, and rarely, perforation or rupture of the SVC resulting in death, have been previously reported.19-22 Along those lines, it is important to note that stent deployment can preclude pacemaker lead extraction,23 central line and/or indwelling catheter removal.
In summary, treating a thrombus-induced SVC syndrome with catheter-directed thrombolysis and/or with stenting could result in few, but substantial complications stressing the need for new strategies that might decrease the morbidity rate.
The Trellis device (Covidien) has been extensively described by our group previously.24 Briefly, it is a 6 or 8 Fr device that allows delivery of t-PA within a specific treatment zone separated from the systemic circulation by 2 balloons. A central wire allows mechanical dispersion of the t-PA within the allocated treatment zone. When a clot is deemed to be the cause of the SVC obstruction, removing it using the Trellis device without systemic intravenous thrombolytic therapy decreases the risk of hematoma, gastrointestinal bleeding, hematemesis, epistaxis, and decreases the length of hospitalization with all the associated cost and comorbidities.
Despite the fact that IPMT followed by stent placement to treat SVC thrombus has been previously described25 with good success, this article stresses the fact that the Trellis device could be used with percutaneous angioplasty without the need for stent deployment as long as there is adequate flow restoration across the stenosis. The latter strategy not only dramatically curtails the cost but also has the potential to decrease the amount of foreign thrombotic material placed in the vessel in patients where symptomatic relief is usually the primary goal. This should also decrease the complication rate that could arise from percutaneous stent placement especially those related to the stent itself such as migration, rethrombosis, SVC rupture, pacemaker lead, and/or indwelling catheter entrapment. In addition, balloon angioplasty for treatment of SVC syndrome has resulted in excellent long-term (more than 6 months) outcomes in almost all reported cases23,26-28 and should be the primary therapy of choice following IPMT in cases of SVC thrombus. However, we do agree with O’Sullivan et al25 that stent placement following IPMT should still be advocated but we think that it should be reserved to patients who fail IPMT and balloon angioplasty and those without indwelling venous lines or pacemaker leads.
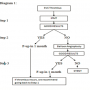
Therefore, we recommend following a staged procedural strategy for treatment of SVC thrombus (Diagram 1). When patients present with SVC thrombus, IPMT should be attempted first (Step 1), followed by balloon angioplasty (Step 2), and then stenting (Step 3), depending on the ultimate results. We define “good result” as less than 30% stenosis seen on the follow-up venogram with less than 5 mm Hg of pressure gradient along with restoration of the respiratory variation in the distal segment. If the repeat angiogram and pressure calculation reveal a good result at any step, the procedure can be aborted with adequate outpatient follow-up in 1 month.
Following the treatment of cancer-related SVC thrombus, we give all our patients life-long low molecular weight heparin despite the fact that the ACCP indicate that the need for such long-term therapy has not been established yet.
Conclusion
Patients with cancer-related SVC thrombus can be adequately treated with the Trellis device using a staged procedural strategy. If no residual thrombus was seen on angiography, stent placement is not needed. This strategy did not affect the short-term patency rate in our patients and seems to be a cost-effective and safe treatment option in such patients with a usually very grim prognosis.
References
- García Mónaco R, Bertoni H, Pallota G, et al. Use of self-expanding vascular endoprostheses in superior vena cava syndrome. Eur J Cardiothorac Surg. 2003 Aug;24(2):208-211.
- Rowell NP, Gleeson FV. Steroids, radiotherapy, chemotherapy and stents for superior vena caval obstruction in carcinoma of the bronchus: a systematic review. Clin Oncol (R Coll Radiol). 2002 OCt;14(5):338-351.
- Kalra M, Gloviczki P, Andrews JC, et al. Open surgical and endovascular treatment of superior vena cava syndrome caused by nonmalignant disease. J Vasc Surg. 2003 Aug;38(2):215-223.
- William H. History of aneurysm of the aorta with some remarks on aneurysm in general. Medical Observations and Inquiries. 1757;323.
- Schechter MM. The superior vena cava syndrome. Am J Med Sci. 1954 Jan;227(1):46-56.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore). 2006 Jan;85(1):37-42.
- Chee CE, Bjarnason H, Prasad A. Superior vena cava syndrome: an increasingly frequent complication of cardiac procedures. Nat Clin Pract Cardiovasc Med. 2007 Apr;4(4):226-230.
- Sivaram CA, Craven P, Chandrasekaran K. Transesophageal echocardiography during removal of central venous catheter associated with thrombus in superior vena cava. Am J Card Imaging. 1996 Oct;10(4):266-269.
- Bell DR, Woods RL, Levi JA. Superior vena caval obstruction: a 10-year experience. Med J Aust. 1986 Dec;145(11-12):566-568.
- Lanciego C, Chacón JL, Julián A, et al. Stenting as first option for endovascular treatment of malignant superior vena cava syndrome. AJR Am J Roentgenol. 2001 Sep;177(3):585-593.
- Kvale PA, Selecky PA, Prakash UB; for the American College of Chest Physicians. Palliative care in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007 Sep;132(3 Suppl):368S-403S.
- National Comprehensive Cancer Network (NCCN) guidelines are available online at www.nccn.org.
- Loeffler JS, Leopold KA, Recht A, Weinstein HJ, Tarbell NJ. Emergency prebiopsy radiation for mediastinal masses: impact on subsequent pathological diagnosis and outcome. J Clin Oncol. 1986 May;4(5):716-721.
- Kee ST, Kinoshita L, Razavi MK, Nyman UR, Semba CP, Dake MD. Superior vena cava syndrome: treatment with catheter-directed thrombolysis and endovascular stent placement. Radiology. 1998 Jan;206(1):187-193.
- Qanadli SD, El Hajjam M, Mignon F, et al. Subacute and chronic benign superior vena cava obstructions: endovascular treatment with self-expanding metallic stents. Am J Roentgenol. 1999 Jul;173(1):159-164.
- Smayra T, Otal P, Chabbert V, et al. Long-term results of endovascular stent placement in the superior caval venous system. Cardiovasc Intervent Radiol. 2001 Nov-Dec;24(6):388-394.
- Barshes NR, Annambhotla S, El Sayed HF, et al. Percutaneous stenting of superior vena cava syndrome: treatment outcome in patients with benign and malignant etiology. Vascular. 2007 Sep-Oct;15(5):314-321.
- Crowe MT, Davies CH, Gaines PA. Percutaneous management of superior vena cava occlusions. Cardiovasc Intervent Radiol. 1995 Nov-Dec;18(6):367-372.
- Nagata T, Makutani S, Uchida H, et al. Follow-up results of 71 patients undergoing metallic stent placement for the treatment of a malignant obstruction of the superior vena cava. Cardiovasc Intervent Radiol. 2007 Sep-Oct;30(5):959-967.
- Martin M, Baumgartner I, Kolb M, Triller J, Dinkel HP. Fatal pericardial tamponade after Wallstent implantation for malignant superior vena cava syndrome. J Endovasc Ther. 2002 Oct;9(5):680-684.
- Smith SL, Manhire AR, Clark DM. Delayed spontaneous superior vena cava perforation associated with a SVC wallstent. Cardiovasc Intervent Radiol. 2001 Jul-Aug;24(4):286-287.
- Ghanem A, Tiemann K, Nickenig G. Gone with the flow: percutanous retrieval of a migrated wallstent trapped in the right ventricle. Eur Heart J. 2009 Mar;30(6):717.
- Kastner RJ, Fisher WG, Blacky AR, Bacon ME. Pacemaker-induced superior vena cava syndrome with successful treatment by balloon venoplasty. Am J Cardiol. 1996 Apr;77(9):789-790.
- Pappy R, Hanna EB, Abu-Fadel MS, Hennebry TA. Isolated pharmacomechanical thrombectomy for the management of chronic DVT. J Interv Cardiol. 2011 Feb;24(1):99-104.
- O'Sullivan GJ, Mhuircheartaigh JN, Ferguson D, Delappe E, O'Riordan C, Browne AM. Isolated pharmacomechanical thrombolysis plus primary stenting in a single procedure to treat acute thrombotic superior vena cava syndrome. J Endovas Ther. 2010 Feb;17(1):115-123.
- Grace AA, Sutters M, Schofield PM. Balloon dilatation of pacemaker induced stenosis of the superior vena cava. Br Heart J. 1991 Apr;65(4):225-226.
- Walpole HT Jr, Lovett KE, Chuang VP, West R, Clements SD Jr. Superior vena cava syndrome treated by percutaneous transluminal balloon angioplasty. Am Heart J. 1988 Jun;115(6):1303-1304.
- Sherry CS, Diamond NG, Meyers TP, Martin RL. Successful treatment of superior vena cava syndrome by venous angioplasty. AJR Am J Roentgenol. 1986 Oct;147(4):834-835.
_________________________________________________
From the University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted August 16, 2011, provisional acceptance given September 6, 2011, final version accepted September 14, 2011.
Address for correspondence: Thomas Hennebry, MB, 920 Stanton L. Young Blvd, Rm WP3010, Oklahoma City, OK 73104, USA. Telephone: (405) 271-4742, ext. 44744. Email: Thomas-Hennebry@ouhsc.edu


















