Successful Thrombectomy for Acute Coronary Thromboembolism During HeartMateII Ventricular Support
Download a PDF of this article.
Abstract: Complications of hemostasis account for the most common adverse events during continuous-flow ventricular assist device support for advanced heart failure. Successful management of acute coronary thromboembolism during HeartMateII device support has not been described in the literature. We present a case of acute coronary thromboembolism of the left main trunk associated with angina and ventricular tachycardia, successfully treated with aspiration and rheolytic thrombectomy and systemic anticoagulation. The postprocedural management of acute coronary syndromes during axial flow ventricular assist device recipients may be complicated by disorders of primary hemostasis and a long-term tendency for bleeding.
J INVASIVE CARDIOL 2013;25(3):E54-E56
Key words: HeartMateII, adverse events, acute coronary occlusion
__________________________________________
Disorders of hemostasis account for the most common adverse events associated with the use of the latest generation of axial flow ventricular assist devices (VADs) for advanced heart failure.1 Adverse thromboembolic events complicate the post-VAD course of 0.3%-4.4% of axial flow mechanical support recipients and require long-term anticoagulation with both vitamin K antagonism and antiplatelet therapy as a primary prevention strategy.2-4 Among other complications related to use of the HeartMateII VAD (HMII; Thoratec, Inc), thrombosis of the aortic root has been described.5,6 Thrombosis of the left main coronary during HMII support has also been described.7 However, the successful non-surgical management of acute coronary occlusion during HMII support has not been described. We present a case of HMII-associated acute coronary occlusion precipitating acute myocardial infarction (AMI) and ventricular arrhythmia.
Case Description
A 57-year-old female with chronic systolic heart failure secondary to a non-ischemic, chemotherapy-induced cardiomyopathy received an axial flow HMII left ventricular assist device 23 months earlier as destination therapy. Her postoperative course was complicated by recurrent follicular lymphoma.
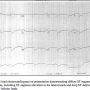 On the day of her acute coronary syndrome (ACS) presentation, the patient was resting in her home when she developed substernal chest pressure followed by discharge of her implantable cardiac defibrillator (ICD). She activated emergency medical services and was transported to a local emergency department. Upon presentation, the patient was hemodynamically stable with intact neurologic status. An electrocardiogram (ECG) showed ominous, diffuse ST-segment deviation (Figure 1). Device interrogation confirmed appropriate ICD discharge for polymorphic ventricular tachycardia. The patient was treated for ACS with aspirin, clopidogrel, and heparin intravenous bolus followed by continuous infusion and emergently referred to the catheterization lab. She was chronically maintained on aspirin 81 mg daily, although was not chronically anticoagulated due to prior major bleeding, including prior ischemic stroke with hemorrhagic conversion, while on HMII support.
On the day of her acute coronary syndrome (ACS) presentation, the patient was resting in her home when she developed substernal chest pressure followed by discharge of her implantable cardiac defibrillator (ICD). She activated emergency medical services and was transported to a local emergency department. Upon presentation, the patient was hemodynamically stable with intact neurologic status. An electrocardiogram (ECG) showed ominous, diffuse ST-segment deviation (Figure 1). Device interrogation confirmed appropriate ICD discharge for polymorphic ventricular tachycardia. The patient was treated for ACS with aspirin, clopidogrel, and heparin intravenous bolus followed by continuous infusion and emergently referred to the catheterization lab. She was chronically maintained on aspirin 81 mg daily, although was not chronically anticoagulated due to prior major bleeding, including prior ischemic stroke with hemorrhagic conversion, while on HMII support.
 Selective coronary arteriography demonstrated a patent, angiographically normal right coronary artery. The left main coronary trunk (LMT) was occluded by a massive saddle embolus, associated with TIMI 0 flow (Figure 2) and preventing distal run-off into the left anterior descending (LAD) and left circumflex (LCX) coronary arteries. With injection of the LMT, extensive thrombus was visualized at the base of the aorta. Because of extensive thrombosis of the aortic root into the LMT, it remained unclear whether surgical exploration would be required; for that reason, a direct thrombin inhibitor or glycoprotein IIb/IIIa inhibitor was avoided. Thrombolysis was relatively contraindicated with a history of prior hemorrhagic stroke. Due to multiple comorbidities, a percutaneous approach was preferred.
Selective coronary arteriography demonstrated a patent, angiographically normal right coronary artery. The left main coronary trunk (LMT) was occluded by a massive saddle embolus, associated with TIMI 0 flow (Figure 2) and preventing distal run-off into the left anterior descending (LAD) and left circumflex (LCX) coronary arteries. With injection of the LMT, extensive thrombus was visualized at the base of the aorta. Because of extensive thrombosis of the aortic root into the LMT, it remained unclear whether surgical exploration would be required; for that reason, a direct thrombin inhibitor or glycoprotein IIb/IIIa inhibitor was avoided. Thrombolysis was relatively contraindicated with a history of prior hemorrhagic stroke. Due to multiple comorbidities, a percutaneous approach was preferred.
Coronary intervention was facilitated with heparin, dosed to a target activated clotting time of >300 seconds. A 6 Fr XB 3.5 guiding catheter was used to engage the LMT, through which a Prowater coronary guidewire (Abbott Vascular, Inc) was advanced into the distal LAD. A second Whisper coronary wire (Abbott Vascular, Inc) was advanced into the distal LCX. Manual thrombectomy was performed with an Export aspiration catheter (Medtronic, Inc) down the LAD, and then LCX. A large amount of thrombus was aspirated from each coronary vessel, successfully restoring TIMI 2 coronary flow. With thrombus clearance incomplete, an AngioJet rheolytic thrombectomy catheter (Medrad, Inc) was sequentially advanced into the LAD and LCX coronary 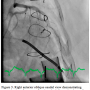 arteries with excellent results. Final angiography (Figure 3) demonstrated TIMI 3 flow to both coronary arteries. After the LAD and LCX vessels were cleared of thrombus, they had angiographically normal contours and were without dissection or atherosclerotic plaque. After the procedure, the patient was continued on aspirin and clopidogrel, and was initiated on warfarin with intravenous heparin bridging. She had no clinical signs of pump malfunction. A transthoracic echocardiogram demonstrated normal ventricular pump inflow and aortic outflow, with no significant aortic valve opening. Pump speed was slightly reduced to increase pulsatility and valve opening. She was managed conservatively with chronic anticoagulation and was discharged 8 days later on aspirin and therapeutic anticoagulation. At time
arteries with excellent results. Final angiography (Figure 3) demonstrated TIMI 3 flow to both coronary arteries. After the LAD and LCX vessels were cleared of thrombus, they had angiographically normal contours and were without dissection or atherosclerotic plaque. After the procedure, the patient was continued on aspirin and clopidogrel, and was initiated on warfarin with intravenous heparin bridging. She had no clinical signs of pump malfunction. A transthoracic echocardiogram demonstrated normal ventricular pump inflow and aortic outflow, with no significant aortic valve opening. Pump speed was slightly reduced to increase pulsatility and valve opening. She was managed conservatively with chronic anticoagulation and was discharged 8 days later on aspirin and therapeutic anticoagulation. At time 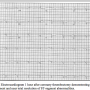 of discharge, her ECG had returned to baseline (Figure 4) and she had no further ventricular arrhythmias. After 60 days of follow-up, she remained well and free of bleeding or thromboembolism.
of discharge, her ECG had returned to baseline (Figure 4) and she had no further ventricular arrhythmias. After 60 days of follow-up, she remained well and free of bleeding or thromboembolism.
Discussion
Acute thromboembolic events during HMII support are primarily associated with ischemic cerebrovascular accidents and rarely pump thrombosis.2,8 Overall, thromboembolism occurs with lower frequency after HMII implant compared to rates of earlier-generation pulsatile ventricular support devices.9 Nevertheless, the non-pulsatile flow during HMII support may uniquely predispose to aortic root thrombosis, owing to a lack of aortic valve opening during significant left ventricular off-loading. Many of these cases may be asymptomatic.6
A report by Freed and colleagues highlighted a case of unsuccessful thrombectomy during aortic root thrombosis that extended into the LMT.7 As described, their patient presented with exertional chest pain similar to prior episodes of angina, atrial fibrillation, and a ventricular-paced rhythm on ECG. Attempts at aspiration and rheolytic thrombectomy were unsuccessful, and the patient was continued on eptifibatide and heparin intravenously, and eventually underwent cardiac transplantation several months later. Our case was unique at presentation, accompanied by ventricular dysrhythmia and acute ECG changes indicating ischemia, likely owing to acute occlusion of the LMT with new thrombus. Reperfusion results with aspiration and rheolytic thrombectomy were excellent, as facilitated by aspirin, clopidogrel, and heparin. Our patient had several comorbidities that prevented her from undergoing eventual cardiac transplantation, although she had a relatively smooth clinical course when continued on aspirin and warfarin long term.
Outside of acute coronary syndromes, sudden massive coronary thromboembolism has been reported in the context of atrial fibrillation10 and thrombosis of mechanical valve prosthesis.11 Reports of thrombectomy in these cases, as among those of VAD recipients, are limited. Nevertheless, thrombectomy likely plays an important role, particularly for acute presentations of coronary occlusion. During more typical cases of ACS, successful thrombectomy has been hypothesized as an important determinant of ST-segment resolution, infarct size, and mortality.12-14 Recently, the Thrombosis Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS) suggested that aspiration of thrombus of the infarct-related artery using a 6 Fr Export aspiration catheter was safe and resulted in improved myocardial blush grade14 and clinical outcome at 1 year15 following primary PCI. Extrapolation of these results to this unique clinical scenario may not be appropriate, although aspiration in this clinical schema did provide an excellent result with restoration of coronary flow. Notwithstanding, this anecdotal evidence may support future use of thrombectomy in the management of massive coronary embolization in a similar clinical scenario.
Conclusion
VAD-associated comorbidities, particularly those related to bleeding and thromboembolism, have tempered the enthusiasm over their use, and can manifest in a variety of clinical scenarios, including acute coronary thromboembolism, as evidenced in the presented case. The presented case is unique, demonstrating the effective use of aspiration and rheolytic thrombectomy, systemic anticoagulation, and adjustment of pump speed to improve pulsatility and aortic valve mobility. Consideration should be given for acute coronary occlusion in the VAD recipient who develops ventricular tachycardia and appropriate defibrillator discharge, even in the absence of significant hemodynamic instability. Coronary thromboembolism associated with HMII support can be safely and effectively managed with aspiration and rheolytic thrombectomy, followed by a maintenance regimen of antiplatelet and antithrombotic therapy.
References
- Eckman PM, John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation. 2012;125(24):3038-3047.
- Boyle AJ, Russell SD, Teuteberg JJ, et al. Low thromboembolism and pump thrombosis with the HeartMate II left ventricular assist device: analysis of outpatient anti-coagulation. J Heart Lung Transplant. 2009;28(9):881-887.
- John R, Kamdar F, Liao K, et al. Low thromboembolic risk for patients with the Heartmate II left ventricular assist device. J Thorac Cardiovasc Surg. 2008;136(5):1318-1323.
- John R, Kamdar F, Eckman P, et al. Lessons learned from experience with over 100 consecutive HeartMate II left ventricular assist devices. Ann Thorac Surg. 2011;92(5):1593-1599; discussion 1599-1600.
- Demirozu ZT, Ho J, Bogaev RC, Lemaire SA, Coselli JS, Frazier OH. Thrombotic occlusion of an aortic-root xenograft during left ventricular assistance. Tex Heart Inst J. 2011;38(1):66-67.
- Khodanerdian RA, Mason NO, Horton SC, et al. Aortic valve/root thrombosis with continuous flow ventricular assist devices. J Heart Lung Transplant. 2008;27:S132-S133.
- Freed BH, Jeevanandam V, Jolly N. Aortic root and valve thrombosis after implantation of a left ventricular assist device. J Invasive Cardiol. 2011;23(4):E63-E65.
- John R, Kamdar F, Liao K, Colvin-Adams M, Boyle A, Joyce L. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg. 2008;86(4):1227-1234; discussion 1234-1235.
- Starling RC, Naka Y, Boyle AJ, et al. Results of the post-U.S. Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol. 2011;57(19):1890-1898.
- Sakai K, Inoue K, Nobuyoshi M. Aspiration thrombectomy of a massive thrombotic embolus in acute myocardial infarction caused by coronary embolism. Int Heart J. 2007;48(3):387-392.
- Nakazone MA, Tavares BG, Machado MN, Maia LN. Acute myocardial infarction due to coronary artery embolism in a patient with mechanical aortic valve prosthesis. Case Report Med. 2010:751857. (Epub 2010 Jun 14).
- Cura FA, Escudero AG, Berrocal D, et al. Protection of distal embolization in high-risk patients with acute ST-segment elevation myocardial infarction (PREMIAR). Am J Cardiol. 2007;99(3):357-363.
- Gick M, Jander N, Bestehorn HP, et al. Randomized evaluation of the effects of filter-based distal protection on myocardial perfusion and infarct size after primary percutaneous catheter intervention in myocardial infarction with and without ST-segment elevation. Circulation. 2005;112(10):1462-1469.
- Svilaas T, Vlaar PJ, van der Horst IC, et al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008;358(6):557-567.
- Vlaar PJ, Svilaas T, van der Horst IC, et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet. 2008;371(9628):1915-1920.
__________________________________________
From the Robert and Suzanne Tomsich Department of Cardiovascular Medicine, Section of
Interventional Cardiology, Heart and Vascular Institute, Cleveland Clinic, Cleveland, Ohio.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted September 26, 2012 and accepted October 3, 2012.
Address for correspondence: Russell Raymond, DO, Robert & Suzanne Tomsich Department of Cardiovascular Medicine, Heart and Vascular Institute, Cleveland Clinic, Mail code J2-3, 9500 Euclid Avenue, Cleveland, OH 44195. Email: raymonr@ccf.org


















