Successful Stenting of Total Left Subclavian Artery Occlusion Post-Coronary Artery Bypass Graft Surgery Using Dual Left Vertebral Artery and Left Internal Mammary Artery Protection
ABSTRACT: We report on a 68-year-old male patient (smoker, chronic obstructive pulmonary disease, diabetic for 25 years) who was admitted with acute pulmonary edema 5 months after undergoing coronary artery bypass grafting (left internal mammary artery to left anterior descending artery, saphenous vein graft to posterior descending artery, and sequential saphenous vein graft to obtuse marginal artery). He had no cardiac enzyme leakage and his left ventricular ejection fraction was > 50% on 2-dimensional echocardiography. He proved to have total calcific left subclavian artery occlusion and retrograde flow in both his left internal mammary artery and his left vertebral artery upon left subclavian angiography performed through the left brachial approach. Angiographic vertebral and coronary-subclavian steals were both supported with clinical manifestations. The patient underwent successful stenting to his left subclavian artery using dual protection to his left vertebral artery with filter protection device and to his left internal mammary artery using simple balloon inflation at its mouth before and after each subclavian artery angioplasty step. Three months later, the patient was free from any cardiac or neurologic complaints.
J INVASIVE CARDIOL 2011;23:E132–E136
__________________________________________
The increased use of the left internal mammary artery (LIMA) for myocardial revascularization during coronary artery bypass grafting (CABG), due to its excellent long-term patency and relative resistance to atherosclerosis, has exposed another type of subclavian steal, termed coronary subclavian steal (CSS), wherein the left subclavian artery is affected with flow-limiting atherosclerosis.
The endovascular approach to supra-aortic lesions has been successful and is now a widely accepted alternative to surgery. Both angioplasty and stenting procedures have a low mortality and morbidity, with good short-term results, despite the fact that long-term results remain inferior to surgery, especially to carotid subclavian bypass grafts.1–4 While the choice of operative or interventional technique is usually determined by the individual risk factors and the anatomic distribution of the disease, the endovascular approach remains the procedure of choice for high-risk patients or for those patients refusing the surgical option.5 The reported neurologic complication rate of subclavian (SC) artery angioplasty ranges from 0.4–4.7%, which can be devastating.2,6 A similar cardiac complication rate applies to post-CABG patients, where fatal myocardial damage could follow any amount of embolization through the LIMA to its supplied myocardial bed.7
After angioplasty to the SC artery, flow within the vertebral artery (VA) and the LIMA does not become anterograde immediately, but rather over a period of 20 seconds to several minutes. This delayed flow reversal serves as a protective mechanism against cerebral embolism during and shortly after angioplasty of a stenotic SC artery.8 In such a scenario, device protection to both the LIMA and VA is mostly not recommended, especially if quick direct stenting to the SC artery is performed. However, in the case of total SC artery occlusion, where pre-dilatation is needed and a short time interval before performing stenting and sometimes post-dilatation is not guaranteed, applying protection to potentially endangered territories looks to be essential. The combined protection of LIMA and VA during occluded SC artery intervention may decrease the embolization rates to both the posterior cerebral circulation and to the myocardial bed as well, and therefore maximize patient safety post-procedure.
To our knowledge, this is the first case in the literature that describes the use of such dual protection during endovascular revascularization of total SC artery occlusion.
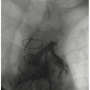 Case Report. We report on a 68-year-old male patient (smoker, chronic obstructive pulmonary disease, diabetic for 25 years) who was admitted with acute pulmonary edema 5 months after undergoing CABG on October 27, 2009 (LIMA to left anterior descending, SVG to posterior descending and sequential SVG to obtuse marginal). The patient admitted past history of dizziness upon moving his neck and left shoulder and arm cramping pains upon using his left arm for the last 2 years with intermittent recent angina.
Case Report. We report on a 68-year-old male patient (smoker, chronic obstructive pulmonary disease, diabetic for 25 years) who was admitted with acute pulmonary edema 5 months after undergoing CABG on October 27, 2009 (LIMA to left anterior descending, SVG to posterior descending and sequential SVG to obtuse marginal). The patient admitted past history of dizziness upon moving his neck and left shoulder and arm cramping pains upon using his left arm for the last 2 years with intermittent recent angina.
Physical examination on admission revealed absent left brachial and radial pulses, while left arm arterial pressure could not be recorded. There was no audible bruit over the left subclavicular area. An electrocardiogram showed old intraventricular conduction defect. Laboratory tests showed no CK-MB leakage with negative two serial troponin-T tests. Echocardiography showed mild anterolateral hypokinesia with estimated left ventricular ejection fraction > 50%. Color Doppler examination of neck vessels documented 30% plaque in the left internal carotid artery and reversal of flow in the left vertebral artery, suggesting total occlusion of the left SC artery.
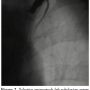 The patient underwent cardiac catheterization by his attending cardiologist, which revealed diffuse native coronary with all vein grafts patent. Patency of the LIMA was demonstrated by retrograde opacification of the vessel during selective catheterization of the LAD as well as by left SC angiography, which also revealed total calcific occlusion of the left SC artery and patent left VA with reversed flow.
The patient underwent cardiac catheterization by his attending cardiologist, which revealed diffuse native coronary with all vein grafts patent. Patency of the LIMA was demonstrated by retrograde opacification of the vessel during selective catheterization of the LAD as well as by left SC angiography, which also revealed total calcific occlusion of the left SC artery and patent left VA with reversed flow.
The patient was subsequently referred by his cardiologist to the vascular surgery team in our hospital, who in turn advised the patient to undergo carotid-subclavian bypass operation, considering angioplasty very high-risk in the presence of adjacent LIMA. The patient decided to seek a second opinion by our interventional team, as he was reluctant to undergo another highly invasive surgery within a short period from his CABG date.
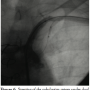
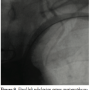
 Procedure. The procedure was performed under local anesthesia. A 6 French (Fr) introducer was placed in the right femoral artery and another 6 Fr was placed in the contralateral brachial artery. Left SC artery angiography demonstrated occlusion of the proximal SC artery and flow reversal in the VA and LIMA (Figures 1 and 2). Both carotid arteries were patent, except for 30% plaque in the left internal carotid artery.
Procedure. The procedure was performed under local anesthesia. A 6 French (Fr) introducer was placed in the right femoral artery and another 6 Fr was placed in the contralateral brachial artery. Left SC artery angiography demonstrated occlusion of the proximal SC artery and flow reversal in the VA and LIMA (Figures 1 and 2). Both carotid arteries were patent, except for 30% plaque in the left internal carotid artery.
Protection to both the VA and LIMA from the expected embolic shower during SC artery angioplasty was achieved by positioning a FilterWire EZ (Boston Scientific Corporation, Natick, Massachusetts) in the proximal part of the left vertebral artery and a 2.5 mm Maverick balloon (Boston Scientific Corporation) in the mouth of the LIMA via the left brachial artery route, after performing systemic heparinization and achieving an activated coagulation time of around 300 seconds.
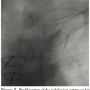
 A 0.014˝ Choice PT graphix guidewire (Boston Scientific Corporation) was passed through the SC lesion via the transbrachial route with some difficulty. The guidewire was then advanced down into the iliac artery and pulled out of the femoral introducer using a GooseNeck snare (ev3, Inc., North Plymouth, Minnesota) (Figures 3 and 4). The wire was then introduced within a 5 Fr, 11 cm pediatric sheath (Cordis Corporation, Miami, Florida). A 5 Fr multipurpose guiding catheter (GC) was later advanced over the 0.014˝ wire and positioned just near the ostium of the left SC artery. The lesion was then predilated with a 2 x 20 mm and then a 3 x 20 mm Maverick balloon (Figure 5). Only then could the GC advance across the distal portion of the vessel to the lesion. At this moment, the 0.014˝ wire was removed and exchanged for a 0.035˝ wire (260 cm in length) through the GC. Then, the 5 Fr introducer femoral sheath was exchanged for a 7 Fr, 61 cm SL2 sheath (St. Jude Medical, Daig Division, Minnetonka, Minnesota) positioned just near the ostium of the left subclavian and subsequently a 7 x 37 mm Express LD stent (Boston Scientific Corporation) was deployed in situ (Figure 6). Advancement of the GC across the lesion after predilation as well as all inflations within the SC artery were protected with prior LIMA 2.5 mm balloon inflation at 6 atm with subsequent deflation 5 seconds after deflating the subclavian balloon or stent. Post-dilatation of the stent was not performed. The LIMA balloon was withdrawn, then the Filterwire was retrieved with its retrieval sheath (Boston Scientific Corporation) through the brachial sheath without any difficulty (Figure 7). The patient tolerated the procedure well and there were no complications. Some debris was retrieved in the Filterwire.
A 0.014˝ Choice PT graphix guidewire (Boston Scientific Corporation) was passed through the SC lesion via the transbrachial route with some difficulty. The guidewire was then advanced down into the iliac artery and pulled out of the femoral introducer using a GooseNeck snare (ev3, Inc., North Plymouth, Minnesota) (Figures 3 and 4). The wire was then introduced within a 5 Fr, 11 cm pediatric sheath (Cordis Corporation, Miami, Florida). A 5 Fr multipurpose guiding catheter (GC) was later advanced over the 0.014˝ wire and positioned just near the ostium of the left SC artery. The lesion was then predilated with a 2 x 20 mm and then a 3 x 20 mm Maverick balloon (Figure 5). Only then could the GC advance across the distal portion of the vessel to the lesion. At this moment, the 0.014˝ wire was removed and exchanged for a 0.035˝ wire (260 cm in length) through the GC. Then, the 5 Fr introducer femoral sheath was exchanged for a 7 Fr, 61 cm SL2 sheath (St. Jude Medical, Daig Division, Minnetonka, Minnesota) positioned just near the ostium of the left subclavian and subsequently a 7 x 37 mm Express LD stent (Boston Scientific Corporation) was deployed in situ (Figure 6). Advancement of the GC across the lesion after predilation as well as all inflations within the SC artery were protected with prior LIMA 2.5 mm balloon inflation at 6 atm with subsequent deflation 5 seconds after deflating the subclavian balloon or stent. Post-dilatation of the stent was not performed. The LIMA balloon was withdrawn, then the Filterwire was retrieved with its retrieval sheath (Boston Scientific Corporation) through the brachial sheath without any difficulty (Figure 7). The patient tolerated the procedure well and there were no complications. Some debris was retrieved in the Filterwire.
 Post-procedural angiography demonstrated a widely patent stent with anterograde flow within the LIMA up to the anastomosis and within the left VA (Figure 8). The distal end of the stent was located at the origin of both the left vertebral and LIMA. After the procedure, the left radial pulse became palpable and was noticeably strong and equal in volume to the right one. The blood pressure difference between the upper limbs disappeared. Three months later, the patient was asymptomatic and there was no upper limb pressure difference.
Post-procedural angiography demonstrated a widely patent stent with anterograde flow within the LIMA up to the anastomosis and within the left VA (Figure 8). The distal end of the stent was located at the origin of both the left vertebral and LIMA. After the procedure, the left radial pulse became palpable and was noticeably strong and equal in volume to the right one. The blood pressure difference between the upper limbs disappeared. Three months later, the patient was asymptomatic and there was no upper limb pressure difference.
Discussion. Atherosclerotic stenosis or obstruction of the SC artery is rare, often asymptomatic and involves the left SC artery 3–4 times more often than the right.9
An early study by Brown suggested that the incidence of significant brachiocephalic disease in patients who undergo elective CABG is 0.5–2.0%.10 Subsequent lower frequency rates were reported, but were later attributed to under-reporting.11,12
 The increased use of the LIMA for myocardial revascularization due to its excellent long-term patency and relative resistance to atherosclerosis has resulted in another type of subclavian steal termed coronary subclavian steal (CSS), in which the steal consists of siphoning of blood from the myocardium through the LIMA graft to the SC artery, resulting in myocardial ischemia to the territory supplied by the graft.13 Coronary subclavian steal was initially described by Harjola and Valle in 1974.14
The increased use of the LIMA for myocardial revascularization due to its excellent long-term patency and relative resistance to atherosclerosis has resulted in another type of subclavian steal termed coronary subclavian steal (CSS), in which the steal consists of siphoning of blood from the myocardium through the LIMA graft to the SC artery, resulting in myocardial ischemia to the territory supplied by the graft.13 Coronary subclavian steal was initially described by Harjola and Valle in 1974.14
The discovery of diseased SC artery in a post-CABG patient means that the LIMA conduit was constructed in the presence of unrecognized, ipsilateral disease, or it more commonly appeared years after surgery as the SC artery disease progressed from mild to severe. Bryan et al15 reported that the mean time from surgery to the report of symptoms was 7.8 years (range: 1 month to 18 years), among 5 patients with post-CABG CSS syndrome.
 On the other hand, subclavian steal syndrome (SSS) refers to SC artery steno-occlusive disease proximal to the origin of the vertebral artery and is associated with flow reversal in the vertebral artery. SSS was first recognized by Contorni and Reivich in the early 1960s.16,17
On the other hand, subclavian steal syndrome (SSS) refers to SC artery steno-occlusive disease proximal to the origin of the vertebral artery and is associated with flow reversal in the vertebral artery. SSS was first recognized by Contorni and Reivich in the early 1960s.16,17
In our case, the patient had both CSS and SSS manifestations, which mandated management. His cardiac symptoms reflect ongoing steal from his LAD territory with resultant major ischemia and his dizzy spells reflect similar vertebro-basilar ischemia.
Angiography of the brachiocephalic arteries at the time of coronary angiography is effective in preventing CSS in a selected group of patients who are candidates for bypass grafting. However, the efficacy of screening the proximal SC artery in all patients undergoing cineangiography of the coronary arteries is still debated.18 Therefore, all patients undergoing CABG should have bilateral pre-operative blood pressure measurement. If the systolic blood pressure between the two arms differs by more than 20 mmHg, such patients should undergo pre-operative angiography of the SC artery.19,20
 CSS has been reported to be successfully managed via thoracotomy with carotid-subclavian,15 axillo-axillary bypass,21 and transposition of the subclavian artery up to the common carotid artery or even to the aorta.13,22,23 Peri-operative mortality is low (0–0.8%), and stroke rate ranges from 0.5–5%. Five-year primary patency rates range from 92–95%, and 8–10-year primary patency ranges from 83–95%.13
CSS has been reported to be successfully managed via thoracotomy with carotid-subclavian,15 axillo-axillary bypass,21 and transposition of the subclavian artery up to the common carotid artery or even to the aorta.13,22,23 Peri-operative mortality is low (0–0.8%), and stroke rate ranges from 0.5–5%. Five-year primary patency rates range from 92–95%, and 8–10-year primary patency ranges from 83–95%.13
Endovascular approaches to supra-aortic lesions have been successful and are now widely accepted alternatives to surgery. Both angioplasty and stenting procedures have a low mortality and morbidity, with good short-term results, despite that they are less durable compared to surgery.1–4
The advantage of crossing a totally occluded SC artery via a transbrachial route lies in its shorter and less tortuous path to the lesion, which provides perfect coaxial positioning of the catheter for crossing and clear visualization of the LIMA and VA ostia. In addition, this route enables insertion of the filter and the balloon without interference with the stenting procedure.
On the other hand, stenting via the transfemoral route avoids placing a large introducer into the brachial artery and allows preservation of the brachial artery for both the filter wire and the LIMA protective balloon. Although the predicted local complications are assumed to be far fewer with a transradial approach, we opted for a transbrachial route for the following reasons: the left radial artery pulsation was absent prior to the procedure, and its diameter was only 1.6 mm with Doppler guidance, which might have hampered the advancement of the 6 Fr sheath necessary to accommodate both the filter and the balloon. An alternative to brachial artery puncture would have been to perform a brachial artery cut down, but that would have added to the complexity of the procedure as well as to the time needed to finish the procedure; a brachial artery cut down would probably have been considered had we failed in our second or third percutaneous brachial punctures.
It is very interesting to point out the technical difficulties that are faced during this approach. The puncture of the hypotensive left brachial artery is difficult and may necessitate the presence of Doppler guidance. Also, the 0.014˝ wire is prone to kink while being dragged by the snare, which creates difficulty in its use at later steps. Furthermore, it should be noted that extreme care should be taken while releasing the distal end of the stent in order not to jail both the filter wire and the LIMA protective balloon. In addition, it should be remembered that the filter wire should be deployed first in the VA to allow enough space to advance the LIMA protective balloon in the brachial sheath. Conversely, the LIMA protective balloon should be withdrawn first at the end of the procedure to allow enough space for advancing the retrieval sheath of the filter wire. By following the above-mentioned sequence, considerable time is gained.
Following SC artery angioplasty, flow within the VA and LIMA does not become anterograde immediately, but rather over a period of 20 seconds to several minutes. This delayed flow reversal serves as a protective mechanism against cerebral embolism during and shortly after angioplasty of a stenotic SC artery.8 In this context, we have three possible scenarios. In the first, a stenotic SC artery lesion can be directly stented. In such a scenario, device protection to both the VA and LIMA arteries is mostly not recommended, especially if quick direct stenting to the SC artery is performed, before the passage of the mentioned 20–30 seconds.24 The second scenario is similar to the first one, but with post-dilatation needed; in this case, protection is usually needed, because the flow may become anterograde prior to post-dilatation. The third scenario (like in our case), includes totally occluded SC artery lesions, where we believe protection is strongly needed because the flow in both the LIMA and VA would become anterograde at several time intervals during angioplasty steps, i.e., at SC artery pre-dilatation, during stenting and post-dilatation. All of the mentioned interventional steps were protected with periodic LIMA balloon inflations, beginning seconds before and ending seconds after any manipulation within the SC arterial lesion.
In contemporary practice, some operators reported successful stenting of totally occluded SC arteries with demonstrated anterograde flow of the LIMA or VA, using balloon protection to the LIMA7 or filter wire for VA protection.25,26 On the contrary, Sadek et al reported stenting of a totally occluded SC artery without the use of any kind of protection.27
Despite the observed efficacy and safety of dual vertebral and LIMA protection during angioplasty to total SC artery occlusion, which was demonstrated in our report, it cannot be proven that such dual protection represents the ideal interventional practice to follow. Further case series or possibly future randomized studies are awaited.
References
- Patel SN, White CJ, Collins TJ, et al. Catheter-based treatment of the subclavian and innominate arteries. Catheter Cardiovasc Interv 2008;71:963–968.
- Angle JF, Matsumoto AH, McGraw JK, et al. Percutaneous angioplasty and stenting of left subclavian artery stenosis in patients with left internal mammary-coronary bypass grafts clinical experience and long-term follow-up. Vasc Endovascular Surg 2003;37:89–97.
- Przewlocki T, Kablak-Ziembicka A, Pieniazek P, et al. Determinants of immediate and long-term results of subclavian and innominate artery angioplasty. Catheter Cardiovasc Interv 2006;67:519–526.
- Sixt S, Rastan A, Schwarzwälder U, et al. Long-term outcome after balloon angioplasty and stenting of subclavian artery obstruction: A single centre experience. Vasa 2008;37:174–182.
- AbuRahma AF, Bates MC, Stone PA, et al. Angioplasty and stenting versus carotid-subclavian bypass for the treatment of isolated subclavian artery disease. J Endovasc Ther 2007;14:698–704.
- Prasad A, Prasad A, Varghese I, et al. Prevalence and treatment of proximal left subclavian artery stenosis in patients referred for coronary artery bypass surgery. Int J Cardiol 2009;133:109–111.
- Nishio A, Takami T, Ichinose T, et al. Percutaneous transluminal angioplasty and stent placement for subclavian steal syndrome with concomitant anterograde flow in the left internal mammary artery graft for coronary artery bypass: Case report. Neurol Med Chir (Tokyo) 2003;43:488–492.
- Ringelstein EB, Zeumer H. Delayed reversal of vertebral artery blood flow following percutaneous transluminal angioplasty for subclavian steal syndrome. Neuroradiology 1984;26:189–198.
- Fields WS, Lemak NA. Joint study of extracranial arterial occlusion. VII. Subclavian steal — A review of 168 cases. JAMA 1972;222:1139–1143.
- Brown AH. Coronary steal by internal mammary graft with subclavian stenosis. J Thorac Cardiovasc Surg 1977;73:690–693.
- Takach TJ, Reul GJ, Duncan JM, et al. Concomitant brachiocephalic and coronary artery disease outcome and decision analysis. Ann Thorac Surg 2005;80:564–569.
- Takach TJ, Reul GJ, Cooley DA, et al. Myocardial thievery: The coronary-subclavian steal syndrome. Ann Thorac Surg 2006;81:386–392.
- Olsen CO, Dunton RF, Maggs PR, Lahey SJ. Review of coronary-subclavian steal following internal mammary artery-coronary artery bypass surgery. Ann Thorac Surg 1988;46:675–678.
- Harjola PT, Valle M. The importance of aortic arch or subclavian angiography before coronary reconstruction. Chest 1974;66:436–438.
- Bryan FC, Allen RC, Lumsden AB. Coronary-subclavian steal syndrome: Report of five cases. Ann Vasc Surg 1995;9:115–122.
- Contorni L. [The vertebro-vertebral collateral circulation in obliteration of the subclavian artery at its origin]. Minerva Chir 1960;15:268–271.
- Reivich M, Holling HE, Roberts B, Toole JF. Reversal of blood flow through the vertebral artery and its effect on cerebral circulation. N Engl J Med 1961;265:878–885.
- Rigatelli G, Rigatelli G. Simultaneous preoperative brachiocephalic angiography and coronary angiography to prevent coronary-subclavian steal syndrome in coronary surgery candidates Heart Surg Forum 2005;8:E175–E177.
- Marshall WG Jr, Miller EC, Kouchoukos NT. The coronary-subclavian steal syndrome: Report of case and recommendation for prevention and management. Ann Thorac Surg 1988;46:193–196.
- FitzGibbon GM, Keon WJ. Coronary subclavian steal, a recurrent case with notes on detecting the threat potential. Ann Thorac Surg 1995;60:1810–1812.
- Iwaki H, Kuraoka S, Tatebe S. Coronary subclavian steal syndrome, report of a case. Kyobu Geka 2003;56:235–238.
- Chung DA, Large SR. Relocation of the internal mammary artery graft in a case of coronary-subclavian steal. Thorac Cardiovasc Surg 2000;48:39–40.
- Penninga L, Damgaard S. [Coronary subclavian steal syndrome: Two cases after coronary artery bypass grafting]. Ugeskr Laeger 2008;70:1158. [Article in Danish].
- Amor M, Eid-Lidt G, Chati Z, et al. Endovascular treatment of the subclavian artery: Stent implantation with or without predilatation. Cathet Cardiovasc Interv 2004;63:364–370.
- Michael TT, Banerjee S, Brilakis E. Subclavian artery intervention with vertebral embolic protection. Catheter Cardiovasc Interv 2009;74:22–25.
- Gimelli G, Tefera G, Turnipseed WD. Vertebral artery embolic protection via ipsilateral brachial approach during left subclavian artery angioplasty and stenting — A case report. Vasc Endovascular Surg 2006;40:235–238.
- Sadek MM, Ravindran A, Marcuzzi DW, Chisholm RJ. Complete occlusion of the proximal subclavian artery post-CABG: Presentation and treatment. Can J Cardiol 2008;24:591–592.
___________________________________
From the Adult Cardiology Department, Queen Alia Heart Institute, Royal Jordanian Medical Services, Amman, Jordan.
The authors report no conflicts of interest regarding the content herein.
Manuscript submitted August 25, 2010, provisional acceptance given September 13, 2010, final version accepted October 25, 2010.
Address for correspondence: Abdallah F. Omeish, MD, Queen Alia Heart Institute, Adult Cardiology Department, PO Box 2251, 11821 Jordan. Email: Abdallah.omeish@yahoo.com


















