Selective Coronary Artery Angiography in Hypoplastic Left Heart Syndrome
Abstract: Coronary artery disease in palliated hypoplastic left heart syndrome is uncommon. Myocardial infarction from a coronary thrombus, serving as a substrate for ventricular arrhythmia in Fontan physiology, is under-reported despite known hypercoagulopathic state. Traditional risk factors for coronary artery occlusion include intracardiac thrombi, hyperlipidemia, and hypertension. Baffle leaks and abnormal ventriculocoronary fistulae found in these patients are contributing factors. We sought to assess and describe coronary artery involvement in this complex patient population. Our research highlights both the need to assess distal coronary vasculature and to thoroughly evaluate hemodynamics and biventricular function with new-onset troponin leak or ventricular arrhythmias.
Key words: coronary thrombus, hypoplastic left heart syndrome, non-sustained ventricular tachycardia, pacemaker
Hypoplastic left heart syndrome (HLHS) is a rare disease, constituting 2%-3% of all congenital heart disease with a prevalence of 3%-4%.1 This complex disease requires intervention beginning in the neonatal period and progressing through a 3-stage palliation to a Fontan procedure, which results in separation of the systemic and pulmonary circulations. Coronary artery abnormalities are known to occur in HLHS, especially in the subset of patients with mitral stenosis (MS) and aortic atresia (AA).2,3 MS/AA variant has a higher mortality in the presence of ventriculocoronary connections.4 Coronary-cameral fistulae, abnormalities of origin, intramural course, and coronary artery hypoplasia have all been described in this subgroup in mostly case reports and autopsy studies.2 Myocardial infarction from a coronary thrombus can serve as a substrate for ventricular arrhythmia and is an under-reported entity in this condition. Coronary thrombosis in the Fontan physiology can have adverse effects on single ventricle function. Additionally, ventricular arrhythmias can arise in the setting of a dilated and dysfunctional systemic right ventricle, with a significant risk of sudden cardiac death. The hypercoagulable state and risk of thromboembolic complications in the Fontan population has been well described, and is likely multifactorial in etiology. We sought to evaluate coronary artery involvement in our HLHS population and to determine if the MS/AA variant was associated with higher coronary artery involvement.
Methods
Design and study population. A retrospective chart review of all cardiac catheterizations performed on patients with HLHS over an 18-year period (between 2001 and 2019) at Cleveland Clinic Children’s Hospital in Cleveland, Ohio was conducted. Patients with other types of single-ventricle physiology, patients without records, patients whose cardiac catheterization studies were done at an outside institution, and patients for whom the cardiac catheterization images were not available for review were excluded. Cardiac catheterizations were performed at different stages during the follow-up period and catheter interventions were performed when indicated after discussion with the multidisciplinary team. In addition to routine cardiac catheterization performed prior to the anticipated surgery, cardiac catheterization was done for any clinical concerns (desaturations, reduced exercise tolerance, increased breathlessness, new-onset ventricular arrhythmias) or problems noted on echocardiography. This study was approved by the institutional review board at Cleveland Clinic Children’s Hospital with a waiver of informed consent.
Results
A total of 88 patients (age range, 0-36 years) underwent cardiac catheterizations over the 18-year period. Thirty-six patients (41%) had MS with aortic stenosis (AS); 16 patients (18%) had MS with AA, and 36 patients (41%) had mitral atresia (MA) with AA. While all patients underwent aortograms as part of diagnostic cardiac catheterization, 11 patients underwent selective coronary angiography for a total of 15 procedures, and 5 patients were noted to have coronary artery abnormalities. Eight percent of patients with MS/AS and 6% of patients with MA/AA had coronary abnormalities noted on selective coronary angiography. Indications for selective coronary artery angiography included symptoms of chest pain and exertional syncope, persistent troponin leak, new-onset ventricular arrhythmias, hemoptysis, and worsening systemic right ventricular function (Table 1).
Two patients with MA/AA had coronary artery abnormalities. One patient presented with hemoptysis and was noted to have numerous coronary collaterals to the left lingular pulmonary artery branches and underwent coil occlusion of right coronary artery (RCA) collateral and 2 mid-LAD collaterals (Figure 1). Another patient undergoing pre-Fontan cardiac catheterization had large coronary artery fistulae to the bronchial circulation and into the pulmonary arteries, which decreased in size on repeat catheterization without intervention after left pulmonary artery was recanalized.
Three patients with MS/AS had coronary artery abnormalities. One patient had myocardial bridge in the mid LAD and had presented with new-onset ventricular ectopy and confusion at 5 years of age (status post Fontan at 4 years old), prompting investigation of the coronary arteries.
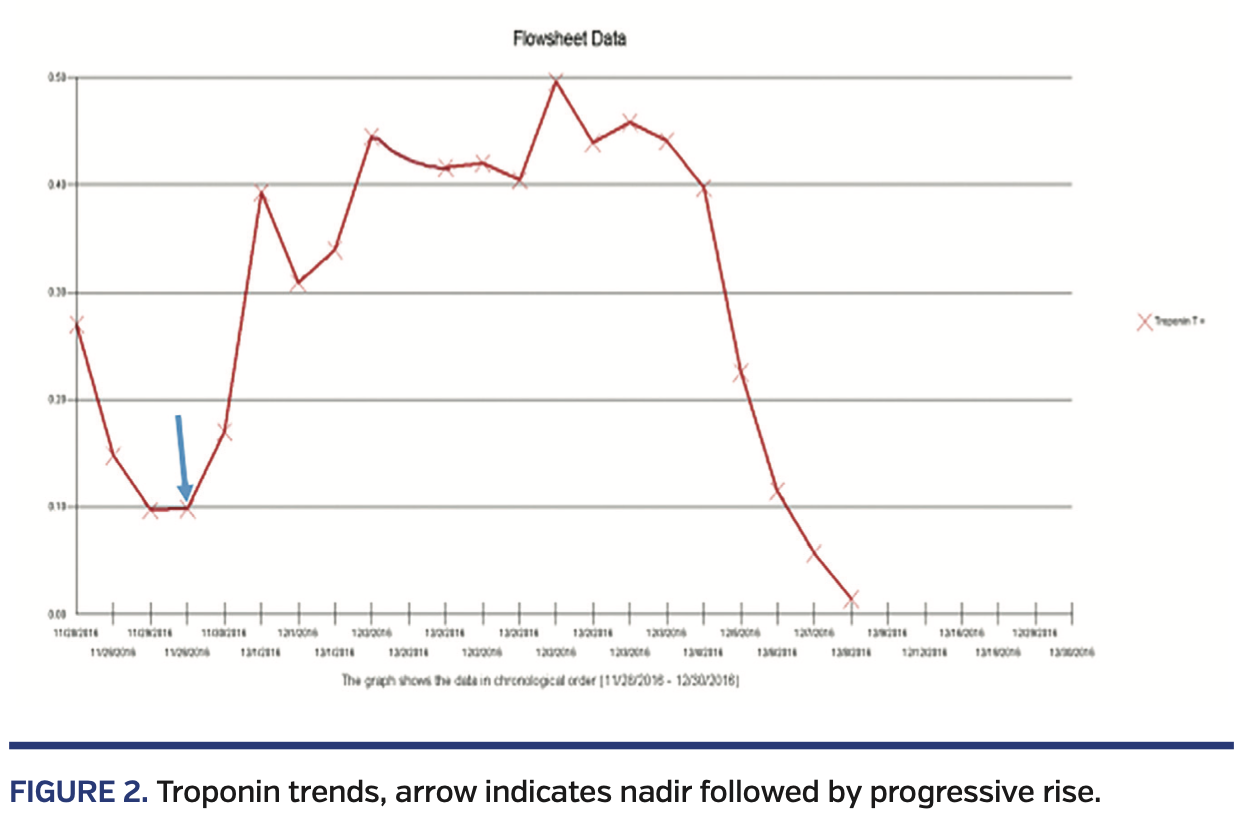
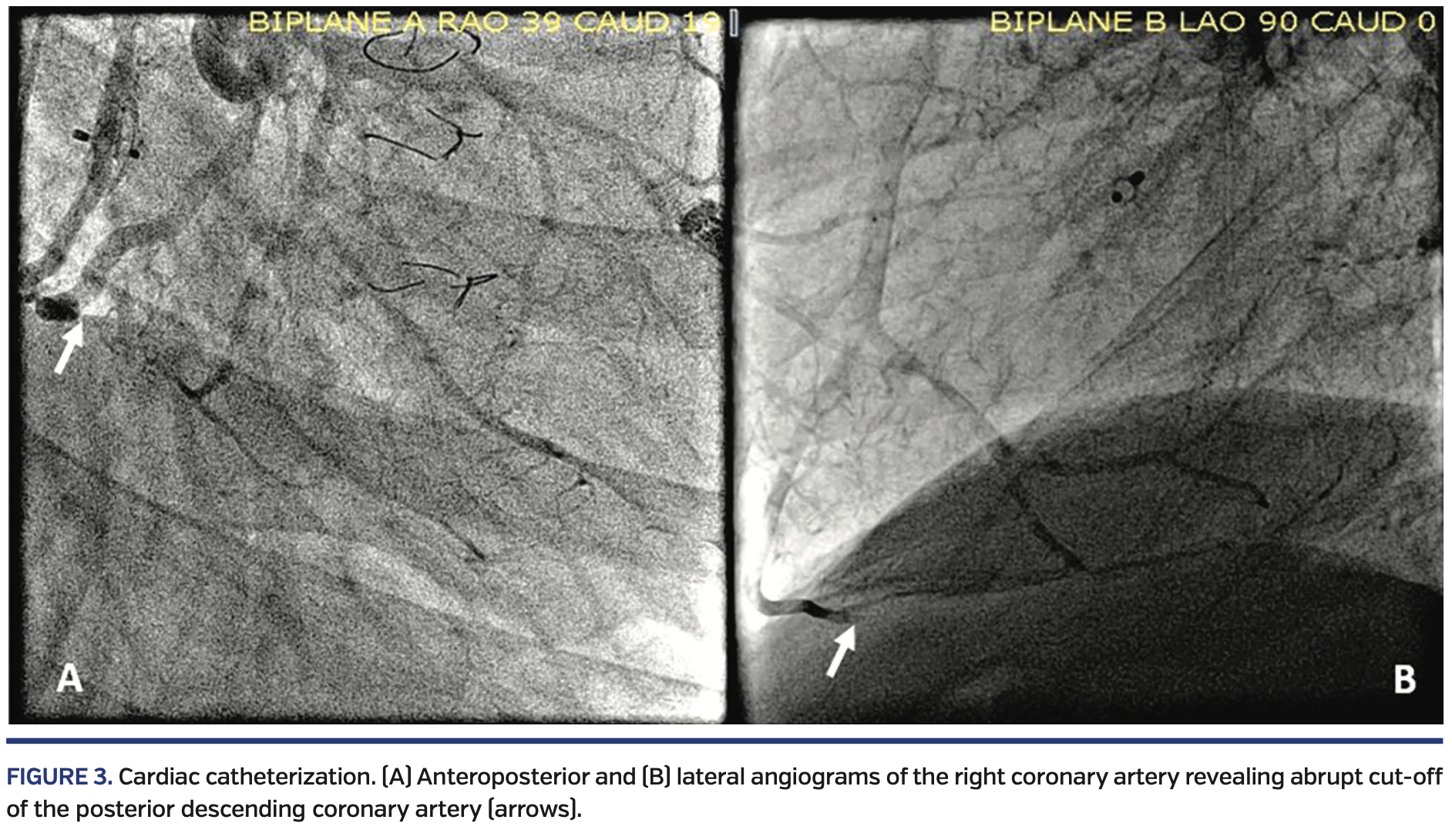
Two other patients with MS/AS had coronary artery occlusion of differing etiology. Both patients were 15 years old at the time of catheterization. One patient with fenestrated lateral-tunnel Fontan procedure, device closure of fenestration, and left pulmonary artery stent presented with non-exertional acute chest pain preceded by upper respiratory symptoms, fever and myalgia, elevated troponin, and electrocardiographic changes. Echocardiogram revealed patent Damus-Kaye-Stansel anastomosis with moderately decreased systolic function. Due to persistent troponin leak (Figure 2) and concern for ischemic process, a cardiac catheterization with selective coronary artery angiography was done. This revealed a right dominant coronary system with a filling defect at the bifurcation of the posterior descending coronary artery (PDA) with preserved distal filling, suggestive of thrombus. The vessel proximal to this bifurcation showed a tubular stenosis or bridge in the distal coronary artery, proximal to the thrombus. An additional small thrombus was noted in the acute marginal branch (Figure 3). He received triple therapy with ticagrelor, tirofiban, and heparin, with resolution of thrombus. He subsequently developed non-sustained ventricular tachycardia necessitating implantable cardioverter defibrillator placement. The other patient, with extracardiac fenestrated Fontan procedure and sinus node dysfunction status post epicardial pacemaker, presented with witnessed syncope and cyanosis. During a diagnostic cardiac catheterization, she developed sustained ventricular fibrillation requiring multiple cycles of defibrillation, cardiopulmonary resuscitation, and antiarrhythmic therapy with amiodarone and lidocaine. Repeat cardiac catheterization a few days later with selective coronary angiography revealed abrupt cut-off of the mid-PDA originating from the RCA, with the distal PDA being filled retrograde by the left coronary system (Figure 4). Positron emission tomography (PET) scan demonstrated a 22%-25% scar of the systemic right ventricle with no evidence of hibernating myocardium. She died a few days later while awaiting transplant. Autopsy demonstrated the epicardial lead impinging on the RCA approximately 2.7 cm distal to its origin. A 6 cm segment of the proximal RCA to the PDA, including the impinged portion, was filled with a dark red blood clot.
Discussion
Our research emphasizes the need to assess coronary artery anatomy in this complex patient population in the presence of predisposing factors. Unlike prior studies, wherein the MS/AA variety was associated with increased mortality and higher incidence of coronary artery abnormalities, in our study, patients with MS/AS had slightly higher incidence of coronary artery abnormalities.15 The lack of pulsatile blood flow to the lungs in the Fontan circulation can be associated with preload-dependent cardiac output in the systemic ventricle and subclinical thromboembolism. Additionally, abnormal platelet activity, as well as elevated circulating levels of protein C, protein S, antithrombin III, and other clotting factor in Fontan patients, indicates the presence of a procoagulant state, with additional risk factors including extensive suture lines, presence of prosthetic material, and sluggish blood flow.5 Thromboembolic phenomena are important long-term complications, because they are associated with significant morbidity and mortality.4,6 However, despite the known hypercoagulable state, coronary artery involvement has thus far been limited to isolated case reports and case series (mostly of autopsy cases).2,3 There are currently no clear guidelines for thrombus prevention in the Fontan population.6-8 Non-compliance with aspirin therapy could have precipitated platelet aggregation and propensity to thrombus formation in 1 of our patients with coronary artery occlusion. Thrombus formation in the native aortic root in patients with HLHS is often fatal, as described by Owens et al.9 Other potential sources of thrombi include the ligated pulmonary artery stump or the rudimentary ventricle.10
Acute coronary embolus causing ST-segment elevation myocardial infarction in a patient with pulmonary atresia and intact ventricular system with known anatomical abnormalities, including a right ventricle to left coronary artery fistula and baffle leak, has been described.11 One of the patients in our study developed thrombus in the absence of traditional risk factors. The differential diagnosis for ST-segment depression with troponin leak includes myocarditis and pericarditis; however, distal coronary artery occlusion must always be assessed, even if the proximal coronary arteries appear normal, as highlighted in 1 of our patients.
The other long-term complication of the Fontan physiology is a multitude of arrhythmias, including intra-atrial re-entrant tachycardia, sinus node dysfunction, and non-sustained ventricular tachycardia associated with ventricular dysfunction. The cardiac anatomy in this patient population poses a unique challenge due to distorted venous pathways, extensive patch material, acquired venous obstruction, underlying hypercoagulable state, abnormal ventricular geometry/position, and inability to access the atria in most cases, needing epicardial systems.12,13 One of our patients had an epicardial pacemaker system for sinus node dysfunction. A recent study by Mah et al demonstrated a higher incidence (5.5%) of coronary compression by epicardial leads despite being relatively rare.14 In their series, symptomatology ranged from no symptoms to fatigue, chest pain alone, chest pain with troponin leak, and sudden cardiac death. All patients in their series underwent surgery, except for 1 detected on autopsy. As multimodality diagnosing strategies continue to evolve, the rate of detection will continue to increase. Risk factors can be mitigated via preventing a strangulating pattern by avoiding multiple loops of the lead overlaying each other and anchoring excess lead material within the pleural space or along the diaphragmatic surface to avoid dislodgment.14 Our second case highlights the importance of looking for potential reversible causes, such as lead impingement on the coronary system, which can be managed surgically if the operative risk is low.
Conclusion
Patients with the MS/AS subtype of HLHS had slightly higher incidence of coronary artery abnormalities noted on selective coronary angiography in our study. Selective coronary angiography was crucial in the appropriate management of this complex patient population and should be strongly considered, especially in patients with new-onset ventricular arrhythmias and troponin leak, as well as those with epicardial pacing systems.
From the 1Department of Pediatric Cardiology, Cleveland Clinic Children’s Hospital, Cleveland, Ohio; 2Nemours/Alfred I. DuPont Hospital for Children, Wilmington, Delaware; 3Children’s Mercy Kansas City, Kansas City, Missouri; and 4Department of Pediatric Cardiology, Nicklaus Children’s Hospital, Miami, Florida.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
The authors report that patient consent was provided for publication of the images used herein.
Final version accepted May 12, 2020.
Address for correspondence: Rukmini Komarlu, MD, Department of Pediatric Cardiology, Cleveland Clinic Children’s Hospital, 9500 Euclid Avenue, Cleveland, OH 44195. Email: komarlr@ccf.org
- Samanek M, Voriskova M. Congenital heart disease among 815,569 children born between 1980 and 1990 and their 15-year survival: a prospective Bohemia survival study. Pediatric Cardiol. 1999;20:411-417.
- Baffa JM, Chen SL, Guttenberg ME, Norwood WI, Weinberg PM. Coronary artery abnormalities and right ventricular histology in hypoplastic left heart syndrome. J Am Coll Cardiol. 1992;20:350-358.
- O’Connor WN, Cash JB, Cottrill CM, Johnson GL, Noonan JA. Ventriculocoronary connnections in hypoplastic left hearts: an autopsy microscopic study. Circulation. 1982:66:1078-1086.
- Khairy P, Fernandes SM, Mayer JE Jr, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117:85-92.
- De Leval MR, Deanfield JE. Four decades of Fontan palliation. Nat Rev Cardiol. 2010;7:520-527.
- Firdouse M, Agarwal A, Chan AK, Mondal T. Thrombosis and thromboembolic complications in Fontan patients: a literature review. Clin Appl Thromb Hemost. 2014;20:484-492.
- McCrindle BW, Manlhiot C, Cochrane A, et al. Factors associated with thrombotic complications after the Fontan procedure: a secondary analysis of a multicenter, randomized trial of primary thromboprophylaxis for 2 years after the Fontan procedure. J Am Coll Cardiol. 2013;61:346-353.
- Alsaied T, Alsidawi S, Allen CC, Faircloth J, Palumbo JS, Veldtman GR. Strategies for thromboprophylaxis in Fontan circulation: a meta-analysis. Heart. 2015;101:173-177.
- Owens ST, Gomez-Fifer C, Ensing GJ. Thrombus formation in the native aortic root in patients with hypoplastic left heart syndrome. Pediatr Cardiol. 2006;27:385-387.
- Lee SY, Baek JS, Kim GB, et al. Clinical significance of thrombosis in an intracardiac blind pouch after a Fontan operation. Pediatr Cardiol. 2012;33:42-48.
- Hastings RS, McElhinney DB, Saric M, Ngai C, Skolnick AH. Embolic myocardial infarction in a patient with a Fontan circulation. World J Pediatr Congenit Heart Surg. 2014;5:631-634.
- Walsh EP, Cecchin F. Arrhythmias in adult patients with congenital heart disease. Circulation. 2007;115:534-545.
- Alexander ME, Cecchin F, Walsh EP, Triedman JK, Bevilacqua LM, Berul CI. Implications of implantable cardioverter defibrillator therapy in congenital heart disease and pediatrics. J Cardiovasc Electrophysiol. 2004;15:72-76.
- Mah DY, Prakash A, Porras D, Fynn-Thompson F, Dewitt ES, Banka P. Coronary artery compression from epicardial leads: more common than we think. Heart Rhythm. 2018;15:1439-1447.
- Siehr SL, Maeda K, Connolly AA, et al. Mitral stenosis and aortic atresia — a risk factor for mortality after the modified Norwood operation in hypoplastic left heart syndrome. Ann Thorac Surg. 2016;101:162-167.