Saphenous Vein Graft Perforation During Percutaneous Coronary Intervention: A Case Series
Download a PDF of this article.
Abstract: Introduction. The outcomes of perforation during aortocoronary saphenous vein graft (SVG) percutaneous coronary intervention (PCI) are poorly studied. Methods. We reviewed all 12 SVG perforations that occurred between November 2005 and November 2011 at two tertiary referral centers. The acute and long-term outcomes of these patients were examined. Results. The perforation was located in the SVG body (n = 6), aortic (n = 3), or distal (n = 3) anastomosis. Most perforations occurred after stent implantation (n = 5) or after stent postdilation (n = 3). The perforation was Ellis class I in 1 patient, II in 3 patients, III in 4 patients, and III with cavity spilling in 3 patients. The perforation spontaneously sealed without requiring further treatment in 3 patients. In the remaining 9 patients, the perforation was treated with prolonged balloon inflation (n = 2) or covered stent implantation (n = 5), but could not be treated in 2 patients who died during the procedure. Seven of the 10 survivors underwent follow-up angiography 5 months to 2 years after the perforation. The target SVG was occluded in 5 patients and had developed severe stenosis in the remaining 2 patients. Conclusions. SVG perforation during PCI carries a high mortality and frequently requires implantation of a covered stent. Perforated SVGs frequently occlude within 2 years post PCI.
J INVASIVE CARDIOL 2013;25(3):157-161
Key words: saphenous vein aortocoronary bypass grafts, SVG occlusion, perforation
_____________________________________
Saphenous vein aortocoronary bypass grafts (SVGs) have high rates of atherosclerosis development and/or occlusion.1 Percutaneous coronary intervention (PCI) is most commonly utilized to treat SVGs with severe luminal obstruction, due to the high risk of repeat coronary artery bypass graft surgery, especially when the left internal mammary graft is patent. However, SVG PCI carries significant risk for complications both during the perioperative period (no reflow and post PCI acute myocardial infarction due to distal embolization) and subsequently (high rates of restenosis or development of new lesions within the target SVG).2 A less common but potentially catastrophic complication of SVG PCI is perforation, which was first described by Drummond in 19873 and which can lead to tamponade and death. Although the overall occurrence of perforations during PCI has been extensively reviewed,4,5 there are limited data on SVG perforation. The goal of the present study was to examine the clinical outcomes and treatment of perforation during SVG PCI among two high-volume tertiary referral centers.
Methods
We identified all patients who developed SVG perforation during SVG PCI between November 2005 and November 2011 at the Montreal Heart Institute in Montreal Canada and at William Beaumont Hospital in Royal Oak, Michigan. The severity of perforation was classified according to the Ellis classification,6 as follows:
Class I: A crater extending outside the lumen only in the absence of linear staining angiographically suggestive of dissection.
Class II: Pericardial or myocardial blush without a ≥1 mm exit hole.
Class III: Frank streaming of contrast through a ≥1 mm exit hole.
Class III-cavity spilling: Perforation into an anatomic cavity chamber, such as the coronary sinus, the right ventricle, etc.
 Patient demographics, periprocedural management strategies, hospital course and angiographic and clinical follow-up data were extracted from the medical records. Continuous parameters were presented as mean ± standard deviation and nominal variables as percentages. All analyses were performed using JMP 8.0 (SAS Institute).
Patient demographics, periprocedural management strategies, hospital course and angiographic and clinical follow-up data were extracted from the medical records. Continuous parameters were presented as mean ± standard deviation and nominal variables as percentages. All analyses were performed using JMP 8.0 (SAS Institute).
Results
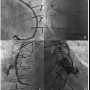
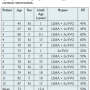 Patients. During the study period, twelve SVG PCIs were complicated by SVG perforation (Figure 1). The clinical characteristics of the patients who developed SVG perforation are shown in Table 1. Most patients (75%) were men presenting with an acute coronary syndrome and mean age was 73 ± 11 years. Mean SVG age was 12 ± 8 years. Most SVG perforations occurred in old SVGs (≥9 years post coronary bypass graft surgery); however, in 3 patients that target SVG was ≤1 year old. Patients who had SVG perforation had extensive native coronary artery and SVG atherosclerosis (Table 2). All except 1 patient had patent left internal mammary grafts to the left anterior descending artery.
Patients. During the study period, twelve SVG PCIs were complicated by SVG perforation (Figure 1). The clinical characteristics of the patients who developed SVG perforation are shown in Table 1. Most patients (75%) were men presenting with an acute coronary syndrome and mean age was 73 ± 11 years. Mean SVG age was 12 ± 8 years. Most SVG perforations occurred in old SVGs (≥9 years post coronary bypass graft surgery); however, in 3 patients that target SVG was ≤1 year old. Patients who had SVG perforation had extensive native coronary artery and SVG atherosclerosis (Table 2). All except 1 patient had patent left internal mammary grafts to the left anterior descending artery.
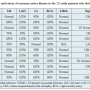 Procedural outcomes. The location of the target SVG lesion was in the SVG body (n = 6), aortic anastomosis (n = 3), or distal anastomosis (n = 3). Most perforations occurred after stent implantation (n = 5) or after stent postdilation (n = 3). In the remaining 4 cases, perforation was caused by lesion predilation, use of an aspiration catheter, use of intravascular ultrasonography, and use of a coronary guidewire. The perforation was Ellis class I in 1 patient, II in 3 patients, III in 4 patients, and III with cavity spilling in 3 patients. The perforation spontaneously sealed without requiring further treatment in 3 patients with class I or II perforation (Table 3). In the remaining 9 patients, the perforation was
Procedural outcomes. The location of the target SVG lesion was in the SVG body (n = 6), aortic anastomosis (n = 3), or distal anastomosis (n = 3). Most perforations occurred after stent implantation (n = 5) or after stent postdilation (n = 3). In the remaining 4 cases, perforation was caused by lesion predilation, use of an aspiration catheter, use of intravascular ultrasonography, and use of a coronary guidewire. The perforation was Ellis class I in 1 patient, II in 3 patients, III in 4 patients, and III with cavity spilling in 3 patients. The perforation spontaneously sealed without requiring further treatment in 3 patients with class I or II perforation (Table 3). In the remaining 9 patients, the perforation was  treated with prolonged balloon inflation (n = 2) or covered stent implantation (n = 5), but could not be treated in 2 patients who died from tamponade during the procedure. All those patients were initially treated with prolonged balloon inflation. That technique was sufficient to seal the perforation in 2 patients, 1 with and 1 without anticoagulation reversal with protamine. Five patients received a covered stent after unsuccessful healing of the perforation with prolonged balloon inflation. Anticoagulation was reversed with protamine in 2 of them, while 1 patient had received bivaluridin. Both patients who died due to an iatrogenic SVG perforation had a class III perforation with cavity spilling. Seven of the 10 survivors underwent follow-up angiography 5 months to 2 years after the perforation. The target SVG was occluded in 5 patients, had developed severe in-stent restenosis in 1 patient, and there was a lesion distal to a covered stent in the last patient. Repeat PCI was performed in 3 of those 7 patients.
treated with prolonged balloon inflation (n = 2) or covered stent implantation (n = 5), but could not be treated in 2 patients who died from tamponade during the procedure. All those patients were initially treated with prolonged balloon inflation. That technique was sufficient to seal the perforation in 2 patients, 1 with and 1 without anticoagulation reversal with protamine. Five patients received a covered stent after unsuccessful healing of the perforation with prolonged balloon inflation. Anticoagulation was reversed with protamine in 2 of them, while 1 patient had received bivaluridin. Both patients who died due to an iatrogenic SVG perforation had a class III perforation with cavity spilling. Seven of the 10 survivors underwent follow-up angiography 5 months to 2 years after the perforation. The target SVG was occluded in 5 patients, had developed severe in-stent restenosis in 1 patient, and there was a lesion distal to a covered stent in the last patient. Repeat PCI was performed in 3 of those 7 patients.
Discussion
The main finding of our study is that SVG perforation carries significant mortality and often requires treatment with covered stent implantation. SVGs that develop perforation often become occluded within 2 years after PCI.
Risk factors and morbidity of SVG perforation. In the United States, SVG interventions represent approximately 6% of the total PCI volume.7 Compared to native coronary artery interventions, SVG PCI is associated with worse in-hospital7 and long-term outcomes.2,4,5 Several factors have been associated with higher risk of perforation, such as coronary calcification, use of cutting or atheroablative devices, treatment of chronic total occlusions, use of intravascular ultrasound, and administration of glycoprotein IIb/IIIa inhibitors.4,5,8
In our series, SVG perforation was a catastrophic event in 2 of 12 patients who developed tamponade and could not be resuscitated. This is consistent with prior reports showing that prior coronary artery bypass graft surgery does not necessarily offer protection from tamponade due to the development of pericardial adhesions.9 Given the often large caliber of SVGs, slightly under-sizing the implanted stent might reduce the risk for rupture and it can also reduce the risk for periprocedural myocardial infarction without compromising long-term patency if drug-eluting stents are used.10
SVG perforation may occasionally have an unusual presentation. A case of SVG perforation into the right atrium has been reported;11 the patient did not develop pericardial effusion or tamponade and was successfully treated with implantation of two covered stents.11 In another case, SVG perforation led to localized pulmonary artery compression.12
Management of SVG perforation. Management of SVG perforation is similar to management of native coronary artery perforation.13 The very first step should be the inflation of a balloon proximal to the perforation site in an attempt to prevent blood extravasation into the pericardial space or the mediastinum. For small perforations, this may suffice; however, if blood extravasation continues, anticoagulation is reversed and a covered stent is deployed,14,15 ideally using a dual guide-catheter technique16 that minimizes the time that blood extravasation occurs while the covered stent is inserted. Although autologous saphenous vein covered stents have been described for the treatment of SVG perforations,17 they are challenging to assemble and deliver, hence manufactured covered stents are preferred. However, only one covered stent, the Jostent Graftmaster (Abbott Vascular) is currently available for use in the United States; the Jostent has a high crossing profile and is challenging to deliver. Other covered stents available in Europe are more flexible and deliverable and have been used to treat SVG perforations, such as the MGuard stent (Inspire-MD), which has an ultra-thin flexible polyethylene theraphthalate (PET or Dacron) mesh sleeve anchored to the external surface of the stent.18,19 Occasionally, coil embolization may be needed in addition to covered stent placement to completely stop the contrast extravasation.20 Although there are reports of perforation sealing using a second (uncovered) stent,21,22 this may be more suitable for small perforations.
If the patient develops hypotension due to tamponade, emergency pericardiocentesis is performed (contrast may enter the pericardial space creating a visible target for the pericardiocentesis needle).13 Emergency surgery may occasionally be the only option for controlling hemorrhage through large SVG perforations;23 however, it can be very challenging due to the time required to start extracorporeal circulation and to enter the chest avoiding injury to other bypass grafts.
Compared to native coronary PCI, SVG PCI failure is associated with high risk for complete vessel occlusion,24 similar to what was observed in our study. In our case series, all the patients with repeat angiography within 2 years after a successfully treated perforation developed disease in the target vessel that led to complete occlusion in most of them. Since perforated SVGs are highly likely to occlude, it may be desirable to plan for alternative forms of revascularization, possibly by recanalization of the native coronary artery that supplies the target coronary segment.25
Study limitations. Although our study is limited by the relatively small number of patients included, it is the only systematic assessment of SVG perforation and importantly, provides for the first time long-term outcomes after sealing of the SVG perforation with covered stents.
Conclusion
In summary, SVG perforation carries high mortality. Prompt recognition and management with balloon inflation, anticoagulation reversal, covered stent implantation, and pericardiocentesis are critical for successful outcomes. Even when successfully sealed, perforated SVGs have high rates of occlusion.
References
- Goldman S, Zadina K, Moritz T, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol. 2004;44(11):2149-2156.
- Lee MS, Park SJ, Kandzari DE, et al. Saphenous vein graft intervention. JACC Cardiovasc Interv. 2011;4(8):831-843.
- Drummer E, Furey K, Hollman J. Rupture of a saphenous vein bypass graft during coronary angioplasty. Br Heart J. 1987;58(1):78-81.
- Al-Lamee R, Ielasi A, Latib A, et al. Incidence, predictors, management, immediate and long-term outcomes following grade III coronary perforation. JACC Cardiovasc Interv. 2011;4(1):87-95.
- Hendry C, Fraser D, Eichhofer J, et al. Coronary perforation in the drug-eluting stent era: incidence, risk factors, management and outcome: the UK experience. EuroIntervention. 2012;8(1):79-86.
- Ellis SG, Ajluni S, Arnold AZ, et al. Increased coronary perforation in the new device era. Incidence, classification, management, and outcome. Circulation. 1994;90(6):2725-2730.
- Brilakis ES, Rao SV, Banerjee S, et al. Percutaneous coronary intervention in native arteries versus bypass grafts in prior coronary artery bypass grafting patients: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv. 2011;4(8):844-850.
- Myles JL, Ratliff NB, Hollman J, Zaidi A, Tan TB. Mechanisms of vessel injury during percutaneous transluminal angioplasty of saphenous vein bypass grafts and coronary arteries. Am J Cardiovasc Pathol. 1988;2(2):133-136.
- Lowe R, Hammond C, Perry RA. Prior CABG does not prevent pericardial tamponade following saphenous vein graft perforation associated with angioplasty. Heart. 2005;91(8):1052.
- Hong YJ, Pichard AD, Mintz GS, et al. Outcome of undersized drug-eluting stents for percutaneous coronary intervention of saphenous vein graft lesions. Am J Cardiol. 2010;105(2):179-185.
- Astroulakis Z, Mathur A. Images in cardiovascular medicine. Percutaneous intervention to a right coronary artery vein graft complicated by perforation into the right heart. Circulation. 2006;114(17):e549-e550.
- Poulter RS, Dooris M. Localized pulmonary artery compression due to saphenous vein graft perforation during percutaneous coronary intervention. Can J Cardiol. 2011;27(3):389.e25-e28.
- Brilakis ES, Karmpaliotis D, Patel V, Banerjee S. Complications of chronic total occlusion angioplasty. Interventional Cardiology Clinics. 2012;1:373-389.
- Latsios G, Tsioufis K, Tousoulis D, Kallikazaros I, Stefanadis C. Perforation of a saphenous vein graft during percutaneous angioplasty: demonstration by means of intravascular ultrasound and consequent treatment with a polytetrafluoroethylene-covered stent. Int J Cardiol. 2009;134(1):e15-e16.
- Baruah DK. Covered stent to treat saphenous venous graft perforation — a case report. Catheter Cardiovasc Interv. 2010;76(6):844-846.
- Ben-Gal Y, Weisz G, Collins MB, et al. Dual catheter technique for the treatment of severe coronary artery perforations. Catheter Cardiovasc Interv. 2010;75(5):708-712.
- Caputo RP, Amin N, Marvasti M, Wagner S, Levy C, Giambartolomei A. Successful treatment of a saphenous vein graft perforation with an autologous vein-covered stent. Catheter Cardiovasc Interv. 1999;48(4):382-386.
- Dziewierz A, Dudek D. Advantages of MGuard coronary stent system. Minerva Cardioangiol. 2012;60(1):33-40.
- Fogarassy G, Apro D, Veress G. Successful sealing of a coronary artery perforation with a mesh-covered stent. J Invasive Cardiol. 2012;24(4):E80-E83.
- Glover B, Johnston P, Spence M. Percutaneous management of a saphenous vein graft perforation using a covered stent and final coil embolization technique. Arch Med Sci. 2010;6(6):991-992.
- Subraya RG, Tannenbaum AK. Successful sealing of perforation of saphenous vein graft by coronary stent. Catheter Cardiovasc Interv. 2000;50(4):460-462.
- Shammas NW, Thondapu VR, Winniford MD, Kalil DA. Perforation of saphenous vein graft during coronary stenting: a case report. Cathet Cardiovasc Diagn. 1996;38(3):274-276.
- Lorusso R, De Cicco G, Ettori F, Curello S, Gelsomino S, Fucci C. Emergency surgery after saphenous vein graft perforation complicated by catheter balloon entrapment and hemorrhagic shock. Ann Thorac Surg. 2008;86(3):1002-1004.
- Lichtenwalter C, de Lemos JA, Roesle M, et al. Clinical presentation and angiographic characteristics of saphenous vein graft failure after stenting: insights from the SOS (stenting of saphenous vein grafts) trial. JACC Cardiovasc Interv. 2009;2(9):855-860.
- Brilakis ES, Banerjee S, Lombardi WL. Retrograde recanalization of native coronary artery chronic occlusions via acutely occluded vein grafts. Catheter Cardiovasc Interv. 2010;75(1):109-113.
____________________________________
From the 1Citizens Memorial Hospital, Heart and Vascular Institute, Bolivar, Missouri, 2William Beaumont Hospital, Department of Cardiology, Royal Oak, Michigan, 3VA North Texas Healthcare System and UT Southwestern Medical Center, Department of Cardiovascular Diseases, Dallas, Texas, 4University of Pittsburgh Medical Center Heart and Vascular Institute, Pittsburgh, Pennsylvania, and 5McGill University Health Center, Interventional Cardiology Department, Montreal, Quebec.
Disclosures: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein. Drs Marmagkiolis, Cilingiroglu, and Hakeem have no disclosures. Dr Brilakis discloses speaker honoraria from St Jude Medical, Terumo and Bridgepoint Medical; grant support to his institution from Guerbet; spouse is an employee of Medtronic. Dr Bilodeau is a BSCI advisory board member.
Manuscript submitted September 19, 2012, provisional acceptance given November 3, 2012, final version accepted November 14, 2012.
Address for correspondence: Konstantinos Marmagkiolis, MD, Citizens Memorial Hospital Heart and Vascular Institute, 1500 North Oakland, Bolivar, MO 65613. Email: c.marmagiolis@gmail.com


















