Saphenous Vein Graft Intervention: A Review
Abstract: Saphenous vein grafts are prone to degeneration and occlusion. Vein graft disease continues to be a significant problem in maintaining long-term benefits after coronary artery bypass surgery. The neointimal hyperplasia and aggressive atherosclerosis that occur in saphenous vein grafts make interventions particularly challenging due to plaque embolization and the no-reflow phenomenon. This review discusses the pathophysiology of vein graft disease and the various percutaneous strategies that have been applied to manage vein grafts. We review the issues surrounding stent selection and various approaches to embolic protection devices. Finally, we discuss the technical steps that optimize success in treating this challenging patient subset.
J INVASIVE CARDIOL 2012;24:64-71
Key words: embolic protection devices, saphenous vein grafts, plaque embolization, no-reflow
_____________________________________
Degeneration and occlusion of saphenous vein grafts (SVG) continue to be significant problems in maintaining long-term benefit in patients who have undergone coronary artery bypass graft (CABG) surgery. SVG occlusion during the first year is high at 15%, and 10-year patency is only 60%.1-4 SVG failure is associated with a significant increase in major adverse cardiovascular events (MACE), including death, myocardial infarction (MI), and the need for repeat revascularization.5 Predictors of vein graft occlusion include tobacco use, hypertension, dyslipidemia, and small target vessel diameter (<2 mm).6 SVG percutaneous coronary intervention (PCI) comprises an important subset of interventions in the cardiac catheterization laboratory. According to the American College of Cardiology National Cardiovascular Data Registry, there were over 90,000 patients (5.7% of all PCIs) who underwent SVG PCI between 2004 and 2009.8
SVG disease occurs in 3 phases: early (before hospital discharge); intermediate (1 month to 1 year); and late (beyond 1 year). Early graft failure is due to thrombotic closure, usually at the site of anastomosis, as a result of endothelial injury and the release of inflammatory cytokines during surgery. Technical factors, such as poor distal runoff, graft kinking, and small target vessel diameter, predispose grafts to early occlusion.6,7 After the first month, exposure of the vein grafts to arterial pressure results in neointimal hyperplasia. This pathophysiologic process causes intimal damage, fibrosis, platelet aggregation, the release of growth factors, and smooth muscle cell proliferation.6 After the first year, aggressive atherosclerotic narrowing occurring over the already abnormal endothelium is the main mechanism for graft failure.
Atherosclerotic plaques in SVGs are more diffuse, friable, contain more foam and inflammatory cells, have absent or small fibrous caps, and little or no calcification in comparison to native coronary atherosclerosis.6 These characteristics predispose SVGs to extensive thrombotic burden and distal embolization during coronary graft interventions, resulting in the no-reflow phenomenon, and hence, more periprocedural MI. Grafts particularly susceptible to these effects are those of an older age with more ectasia and greater plaque burden.9
SVG PCI
Various strategies have been applied in the treatment of patients with SVG failure. Redo-CABG surgery is associated with a marked increase in morbidity and mortality compared with initial surgery10-12 and is therefore used as a last resort. Percutaneous transluminal coronary angioplasty (PTCA) alone also proved to be inadequate therapy with unacceptably high rates of restenosis and MACE.13-15
The Saphenous Vein De Novo (SAVED) trial was the seminal study that compared balloon angioplasty with bare-metal stents (BMS) in SVG lesions.16 This demonstrated that the use of BMS had a better composite outcome of freedom from death, MI, repeat CABG, and target lesion revascularization (TLR).
The advent of drug-eluting stents (DES) dramatically reduced restenosis in native coronaries. With the high rates of restenosis in SVGs,17 DES were also applied in the treatment of SVG stenosis.
Some observational studies comparing DES to BMS in SVG PCI suggest that DES was associated with reduction in TLR and death.18-21 Other studies, however, showed no difference between DES and BMS in terms of death, MI, and target vessel revascularization (TVR).22-25
Randomized prospective studies comparing BMS to DES in SVG PCI are less conclusive because of the small number of studies available and their small sample size. The Reduction in Restenosis in Saphenous Vein Grafts with Cypher (RRISC) trial and the Stenting of Saphenous Vein Graft (SOS) trial compared DES to BMS and found a significant reduction in restenosis and TLR, but no difference in mortality.26,28 RRISC also found a reduction in TVR. However, at 3-year follow-up from the RRISC trial (DELAYED RRISC), there were more deaths in the DES compared with the BMS group.27 In addition, the decrease in TVR seen at 6 months was not noted at follow-up. In contrast, a 3-year follow-up from the SOS trial demonstrated continued benefit with DES, with lower rates of MI and target vessel failure as well as a trend toward less stent thrombosis.29 There were no differences in all-cause mortality or cardiac mortality. Recent meta-analyses have shown that DES had lower TVR and TLR in the observational studies, but this was not confirmed in the randomized studies.30-35
The moderate VEin graft LEsion stenting with the Taxus stent and Intravascular ultrasound (VELETI) trial poses an interesting consideration in the future management of SVG disease. This study showed that stenting moderate SVG lesions with DES showed better luminal area, no progression to occlusion, and a trend toward lower incidence of MACE compared to medical treatment alone.36 A 3-year follow-up confirmed that the incidence of MACE was significantly lower in the stented group.37 Further studies are required to determine if this preventive approach leads to long-term benefit.
Pharmacologic and Mechanical Strategies to Minimize Complications During SVG PCI
The increased incidence of plaque embolization and platelet aggregation presents unique and significant procedural challenges during SVG intervention.38,39 Plaques that develop in SVG are friable and bulky, making them technically difficult during interventions. Vein grafts also have no side branches, and plaque embolization often leads to “slow-flow” or “no-reflow” phenomena where there is diminished or loss of antegrade blood flow to the distal vasculature without angiographic evidence of obstruction. The exact mechanism of the no-reflow phenomenon is unclear, but it is thought to be associated with endothelial swelling, neutrophil infiltration, and platelet aggregation causing obstruction and spasm in the microvasculature.40,41
Various pharmacological and mechanical strategies have been developed in an attempt to decrease complications in SVG PCI. Some of the pharmacologic strategies that have been utilized include the use of glycoprotein (GP) IIb/IIIa inhibitors and vasodilators.
GP IIb/IIIa inhibitors. Adjunctive treatment with platelet GP IIb/IIIa inhibitors in primary PCI for acute ST-elevation myocardial infarction (STEMI) has been shown to improve epicardial blood flow and microvascular perfusion, along with decreasing mortality.42,43 However, the same benefits with GP IIb/IIIa inhibitors were not observed in SVG interventions. One of the reasons for this might include the sheer excessive atheroembolic and thrombotic burden present during SVG interventions. No clinical benefit in terms of reduction in MACE was seen in 2 retrospective studies44,45 and a pooled analysis of 5 studies.46 While the Evaluation of IIb/IIa platelet receptor antagonist 7E3 in Preventing Ischemic Complication (EPIC) trial did find a reduction in the rate of distal embolization and a trend toward reduction in early large non-Q wave MI in patients treated with GP IIb/IIIa inhibitors, the 30-day and 6-month clinical endpoints were similar in both groups.47 A post hoc analysis of the FilterWire EX Randomized Evaluation (FIRE) trial77 showed that GP IIb/IIIa inhibitors in conjunction with FilterWire embolic protection device had better outcomes, with better flow through the filter, and reduced procedural ischemia, as well as less abrupt closure, no reflow, or distal embolization.48 However, similar results were not seen with GP IIb/IIIa inhibitors and the PercuSurge GuardWire embolic protection system in the Saphenous Vein Graft Angioplasty Free of Emboli Randomized (SAFER) trial.75 In fact, in this trial, the patients who were preselected to receive GP IIb/IIIa inhibitors had a higher incidence of MACE. The reason for this increased incidence of MACE may partly be due to selection bias, as operators gave GP IIb/IIIa inhibitors to higher-risk patients who had the most unfavorable lesion morphologies. To date, there are no prospective randomized trials clearly demonstrating the benefits of GP IIb/IIIa inhibitors in SVG PCI. However, while GP IIb/IIIa inhibitors have not been shown to reduce mortality or myonecrosis in SVG PCI, there may be a role in their usage as adjuncts with certain embolic protection devices, such as the distal filtration devices.
Vasodilators. Vasodilators that have been studied in the no-reflow phenomenon include adenosine, verapamil, and nicardipine. Adenosine is a very short-acting, endogenous nucleoside that vasodilates arteries and arterioles and prevents platelet aggregation and thrombus formation. Pretreatment with intracoronary adenosine has been shown to decrease the incidence of MI after elective PCI.49,50 Intracoronary adenosine has also been studied in acute MI and was found to improve myocardial flow51,52 and lower the incidence of the no-reflow phenomenon51,53 and reduce CK elevation.51 The Acute Myocardial Infarction Study of Adenosine (AMISTAD) trials showed adjunctive adenosine infusion reduced the infarct size in patients with anterior ST-elevation MI.54,55
Although there are some data showing benefits of adenosine in elective PCI and acute MI, there are only limited data on the use of adenosine in SVG PCI. There are no studies confirming that adenosine prevents no-reflow, but there are a few small studies showing that adenosine aids in reversing the no-reflow phenomenon. Fischell et al showed promising results with adenosine in reversing slow-flow and no-reflow phenomenon among patients undergoing SVG PCI.56 This finding was later confirmed when repeated boluses of high-dose adenosine reversed no-reflow and improved final Thrombolysis In Myocardial Infarction (TIMI) flow grade.57
Calcium-channel blockers also have been shown to help no-reflow in both laboratory animal models58,59 and clinical trials.60-62 One randomized study, the Vasodilator Prevention of No-Reflow (VAPOR) trial, showed that there was a significant reduction in no-reflow and a trend toward improvement in TIMI flow grade with prophylactic intragraft administration of verapamil during SVG PCI.63 However, this was a small study involving only 22 patients. Two other studies showed that intragraft verapamil successfully treated no-reflow in SVG interventions.64,65 Although verapamil aids in no-reflow, there is no evidence that it protects against MI.66
Nicardipine is another potent calcium-channel blocker that has been shown to be effective in aiding no-reflow. Fugit et al compared 3 intracoronary vasodilators (nicardipine, diltiazem, and verapamil) on nonsignificant native coronary artery disease and found that nicardipine was the most potent coronary vasodilator with the fewest systemic side effects.61 In a retrospective study, nicardipine was successful in reversing 98% of no-reflow episodes without any hemodynamic compromise.67 Following this, Fischell et al showed promising results with nicardipine to prevent no-reflow in SVG PCI.68 They found that pretreatment with intragraft nicardipine, even without the use of mechanical embolic protection, resulted in low incidence of no-reflow and in-hospital MACE.68 Although this study did not have a control group available for direct comparison, nicardipine appeared to be clinically beneficial compared to historical control data66,69 where SVG PCI was done without nicardipine or embolic protection device. Given the ease of administration and cost-effectiveness of nicardipine, the authors concluded that nicardipine might be an alternative or adjunct to mechanical protection devices in SVG PCI.68
Although the available data are limited and not enough to support the routine use of adjunctive pharmacotherapy in SVG PCI, the above studies are promising. Currently, aspirin, thienopyridine, and anticoagulation with heparin are the only recommended adjuvant pharmacotherapy in SVG interventions.
Covered Stents
Covered stents were developed as a mechanical strategy to serve as a local filter, trapping plaque against the graft wall to prevent the shower of emboli during stent deployment. In addition, it was hypothesized that neointimal proliferation and the ensuing restenosis would be reduced. Favorable results were initially suggested by a multicenter registry,70 but randomized trials failed to show any superiority over BMS.71-73 The Stents IN Grafts (STING) trial showed that death, MI, and TLR were comparable in the polytetrafluoroethylene (PTFE)-membrane covered stents versus conventional stents.71 The Randomized Evaluation of polytetrafluoroethylene COVERed stent in Saphenous vein grafts (RECOVERS) trial also demonstrated similar restenosis rates and clinical outcomes between PTFE-covered stents and BMS with higher incidence of nonfatal MI in the PTFE-covered stent group.72 The Symbiot III trial further confirmed that there was no advantage of the Symbiot PTFE-covered stent over BMS in terms of restenosis rates and clinical outcomes.73 The more recent Barrier Approach to Restenosis: Restrict Intima to Curtail ADverse Events (BARRICADE) trial in fact demonstrated more target vessel failure in patients treated with covered stents compared to BMS.74
Embolic Protection Devices
Mechanical embolic protection devices (EPD) were developed and proved to be the first treatment modality to reduce MACE during SVG PCI. Currently, there are 3 types of EPDs: the distal balloon occlusion/aspiration system; distal filter system; and the proximal occlusion/aspiration system.
 Distal occlusion/aspiration system. The PercuSurge GuardWire system occludes the target vessel several centimeters distal to the target lesion during SVG PCI in order to provide myocardial protection. After the intervention, aspiration removes debris-laden blood prior to balloon deflation and restoration of antegrade blood flow (Figure 1).
Distal occlusion/aspiration system. The PercuSurge GuardWire system occludes the target vessel several centimeters distal to the target lesion during SVG PCI in order to provide myocardial protection. After the intervention, aspiration removes debris-laden blood prior to balloon deflation and restoration of antegrade blood flow (Figure 1).
This was the first EPD to gain Food and Drug Administration (FDA) approval following the results of the Saphenous Vein Graft Angioplasty Free of Emboli Randomized (SAFER) trial.75 This pivotal study showed a remarkable 42% reduction in 30-day MACE and a marked decrease in the no-reflow phenomenon with utilization of EPD.
Following the SAFER trial, the TriActiv system was approved by the FDA after proving its noninferiority in the Protection During Saphenous Vein Graft Intervention to Prevent Distal Embolization (PRIDE) trial.76
 Distal filtration system. The FilterWire EX Randomized Evaluation (FIRE) study showed noninferiority of the FilterWire EX System to the GuardWire balloon occlusion and aspiration system and led to FDA approval of the first distal filtration device.77 The FilterWire EX is a guidewire filtration system that uses an oval windsock-shaped filter membrane that is delivered to a “landing zone” distal to the target lesion and then deployed prior to lesion intervention. The intervention is then performed over the wire and a sheath is advanced to retrieve the wire and the filter (Figure 2).
Distal filtration system. The FilterWire EX Randomized Evaluation (FIRE) study showed noninferiority of the FilterWire EX System to the GuardWire balloon occlusion and aspiration system and led to FDA approval of the first distal filtration device.77 The FilterWire EX is a guidewire filtration system that uses an oval windsock-shaped filter membrane that is delivered to a “landing zone” distal to the target lesion and then deployed prior to lesion intervention. The intervention is then performed over the wire and a sheath is advanced to retrieve the wire and the filter (Figure 2).
A newer generation of filter devices has since been developed. The Embolic Protection Transluminally with the FilterWire EZ Device in Saphenous Vein Grafts (BLAZE I and II) study showed a decrease in MACE with FilterWire EZ, a second generation of the FilterWire EX78 and the Saphenous Vein Graft protection In a Distal Embolic Protection Randomized Trial (SPIDER) study showed noninferiority of the Spider Rx filtration device to GuardWire and FilterWire.79 The Assessment of the Medtronic AVE Interceptor Saphenous Vein Graft Filter System (AMEthyst) trial examined another filter, the Interceptor PLUS, which was shown to be noninferior to the GuardWire and FilterWire EZ.80
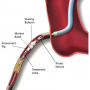 Proximal occlusion/aspiration system. Proximal occlusion devices occlude the vessel proximal to a target lesion and suspend antegrade flow. As with distal occlusion devices, the stagnant blood and debris are then aspirated. The only FDA-approved proximal occlusion/flow reversal device is the Proxis system (Figure 3). This was based on the Proximal Protection During Saphenous Vein Graft Intervention Using the Proxis Embolic Protection System (PROXIMAL) trial that showed the Proxis system to be noninferior to distal EPD.81
Proximal occlusion/aspiration system. Proximal occlusion devices occlude the vessel proximal to a target lesion and suspend antegrade flow. As with distal occlusion devices, the stagnant blood and debris are then aspirated. The only FDA-approved proximal occlusion/flow reversal device is the Proxis system (Figure 3). This was based on the Proximal Protection During Saphenous Vein Graft Intervention Using the Proxis Embolic Protection System (PROXIMAL) trial that showed the Proxis system to be noninferior to distal EPD.81
 The American College of Cardiology guidelines give a class I recommendation for the use of EPD in SVG PCI whenever feasible.82 Despite this, registry data show that EPDs are only utilized in 22% of SVG PCI.83 This may be due to anatomic difficulties, such as challenging take-off from the aorta, very large vessel diameter, and the absence of an adequate non-diseased landing zone. EPD use may also be limited due to the higher procedural cost, longer procedural time, and greater radiation exposure.83 The advantages and disadvantages of each type of EPD are summarized in Table 1.
The American College of Cardiology guidelines give a class I recommendation for the use of EPD in SVG PCI whenever feasible.82 Despite this, registry data show that EPDs are only utilized in 22% of SVG PCI.83 This may be due to anatomic difficulties, such as challenging take-off from the aorta, very large vessel diameter, and the absence of an adequate non-diseased landing zone. EPD use may also be limited due to the higher procedural cost, longer procedural time, and greater radiation exposure.83 The advantages and disadvantages of each type of EPD are summarized in Table 1.
Practical Approaches to SVG PCI
SVG angiography should be undertaken with knowledge of the operative report and any prior angiograms. It is necessary to know the number, location, and anatomy of grafts. This reduces contrast load, radiation exposure, and vascular complications that may occur when blindly searching for graft ostia. Appropriate catheter selection for angiography can also reduce complications and procedural time.
Recently, noninvasive coronary angiography with multidetector computed tomographic (MDCT) angiography has shown promising results. Saphenous vein grafts are great targets for visualization with MDCT because they have large lumens (4 to 6 mm) and reduced overall motion.84 They also can help define vein and arterial graft anatomy. A study by Schlosser et al showed that MDCT had a sensitivity of 96% and a specificity of 95% in evaluation of graft patency.85 MDCT is limited, however, in the visualization of distal anastomosis sites and segments with adjacent clips. Invasive coronary angiography still remains the gold standard for visualization, diagnosis, and treatment of SVG disease.
 Figure 4 illustrates the usual arrangement of vein grafts and the optimal guide catheter selection. The position and angle of graft take-off combined with the size of the aorta determines the type and width of catheter that is appropriate for easy engagement. A vein graft to the right coronary artery often can be engaged by using the Multipurpose catheter, especially if there is a steep inferior take-off. Alternatively, a Judkins Right (JR) catheter or an Amplatz Left (AL) catheter may be used.
Figure 4 illustrates the usual arrangement of vein grafts and the optimal guide catheter selection. The position and angle of graft take-off combined with the size of the aorta determines the type and width of catheter that is appropriate for easy engagement. A vein graft to the right coronary artery often can be engaged by using the Multipurpose catheter, especially if there is a steep inferior take-off. Alternatively, a Judkins Right (JR) catheter or an Amplatz Left (AL) catheter may be used.
Grafts to the left coronary artery can also be engaged by JR catheter, especially if they have a horizontal take-off from the aorta. Alternate catheters include the Left Bypass catheter and the Hockey Stick catheter. Superiorly directed grafts may require an Internal Mammary Artery catheter or an AL catheter.
Once SVG disease is diagnosed and an intervention is required, PCI of the bypassed native vessel should always be considered whenever feasible, as complications of native PCI are lower than that of SVG PCI. If SVG PCI cannot be avoided, the importance of adequate guide support cannot be overemphasized, since safe delivery and retrieval of EPDs need to be taken into account.
Given the current guidelines regarding EPD use,82 we recommend that every effort should be made to use EPDs in SVG interventions whenever technically feasible. The location of the stenosis determines the type of EPD that may be utilized; ostial lesions require a distal EPD, lesions in the body of the graft can be served either by a proximal or distal device, and distal lesions can only be proximally protected. As discussed previously, the addition of intragraft vasodilators should also be considered to aid in the prevention or treatment of no-reflow phenomenon.
The EPD is carefully placed in a satisfactory position. Severe stenoses require predilation with a small balloon. The stent is sized one to one, and high-pressure inflation is avoided to prevent a “cheesegrater effect,” which can increase risk of distal embolization. Minimizing post-stent manipulation is also important to prevent embolization. Both balloon and stent must be well enough away from the EPD to avoid entanglement. Angiography is then repeated to assess the lesion itself and the flow rates in both the vein grafts and the coronary arteries. The myocardial blush grade is also carefully assessed. Slow flow may indicate a packed filter, which should be aspirated and removed. If there is a good angiographic result, the protection balloon is deflated or the filter retrieved. This is a critical step requiring coaxial alignment of the guide to prevent filter entrapment in the stent. A final angiogram is performed to assess the results of the intervention, including the presence of myocardial blush and angiographic flow down the vessel.
Conclusion
SVG PCI is a potentially risk-prone procedure associated with a poor long-term prognosis. Pan-arterial revascularization procedures or a hybrid native coronary stenting with arterial revascularization should be considered to minimize the need for vein grafts.
However, because the use of SVGs is often unavoidable, strict risk factor modifications are important in preventing SVG stenosis. Early aspirin therapy after CABG has improved outcomes including reduction of SVG stenosis and death87,88 and aggressive lipid therapy has also been shown to reduce progression of atherosclerosis in post-CABG patients.89,90 When diseased SVGs require intervention, extreme care must be given in the selection of the stent and the EPD, along with application of good procedural techniques to minimize complications.
References
- Campeau L, Enjalbert M, Lesperance J, et al. The relation of risk factors to the development of atherosclerosis in saphenous vein bypass grafts and the progression of disease in the native circulation: a study 10 years after aortocoronary bypass surgery. N Engl J Med. 1984;311(21):1329-1332.
- Bourassa MG. Fate of venous grafts: the past, the present, and the future. J Am Coll Cardiol. 1991;5(5):1081-1083.
- Fitzgibbon GM, Kafka HP, Leach AJ, et al. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5065 grafts related to survival and reoperation in 1388 patients during 25 years. J Am Coll Cardiol. 1996;28(3):616-626.
- Goldman S, Zadina K, Moritz T, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol. 2004;44(11);2149-2156.
- Halabi AR, Alexander JH, Shaw LK, et al. Relation of early saphenous vein graft failure to outcomes following coronary artery bypass surgery. Am J Cardiol. 2005;96(9):1254-1259.
- Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97(9):916-931.
- Cooper GJ, Underwood MJ, Deverall PB. Arterial and venous conduits for coronary artery bypass. Eur J Cardiothorac Surg. 1996;10(2):129-140.
- Brilakis ES, Wang Ty, Rao SV, et al. Frequency and predictors of drug-eluting stent use in saphenous vein bypass graft percutaneous coronary interventions: a report from the American College of Cardiology National Cardiovascular Data CathPCI registry. JACC Cardiovasc Interv. 2010;3(10):1068-1073.
- Coolong A, Baim DS, Kuntz RE, et al. Saphenous vein graft stenting and major adverse cardiac events: a predictive model derived from a pooled analysis of 3958 patients. Circulation. 2008;117(6):790-797.
- Cameron A, Kemp Jr HG, Green GE. Reoperation for coronary artery disease: 10 years of clinical follow-up. Circulation. 1988;78(3 Pt 2):1158-1162.
- Loop FD, Lytle BW, Cosgrove DM, et al. Reoperation for coronary atherosclerosis: changing practice in 2509 consecutive patients. Ann Surg. 1990;212(3):378-386.
- Christenson JT, Schmuziger M, Simonet F. Reoperative coronary artery bypass procedures: risk factors for early mortality and late survival. Eur J Cardiothorac Surg. 1997;11(1):129-133.
- de Feyter PJ, van Suylen RJ, de Jaegere PP, et al. Balloon angioplasty for the treatment of lesions in saphenous vein bypass grafts. J Am Coll Cardiol. 1993;21(7):1539-1549.
- Morrison DA, Crowley ST, Veerakul G, et al. Percutaneous transluminal angioplasty of saphenous vein grafts for medically refractory unstable angina. J Am Coll Cardiol. 1994;23(5):1066-1070.
- Plokker HW, Meester BH, Serruys PW. The Dutch experience in percutaneous transluminal angioplasty of narrowed saphenous veins used for aortocoronary arterial bypass. Am J Cardiol. 1991;67(5):361-366.
- Savage MP, Douglas JS Jr, Fischman DL, et al. Stent placement compared with balloon angioplasty for obstructed coronary bypass grafts. Saphenous Vein De Novo Trial Investigators. N Engl J Med. 1997;337(11):740-747.
- Hiscock M, Oqueli E, Dick R. Percutaneous saphenous vein graft intervention — a review. Heart Lung Circ. 2007;(16 Suppl 3):S51-S55.
- Ge L, Lakovou L, Sangiorgi GM, et al. Treatment of saphenous vein graft lesions with drug eluting stents: immediate and midterm outcome. J Am Coll Cardiol. 2005;45(7):989-994.
- Lee MS, Shah AP, Aragon J, et al. Drug-eluting stenting is superior to bare-metal stenting in saphenous vein grafts. Catheter Cardiovasc Interv. 2005;66(4):507-511.
- Ramana RK, Ronan A, Cohoon K, et al. Long-term clinical outcomes of real-world experience using sirolimus-eluting stents in saphenous vein graft disease. Catheter Cardiovasc Interv. 2008;6(7):886-893.
- Assali A, Raz Y, Vaknin-Assa H, et al. Beneficial 2-years results of drug-eluting stents in saphenous vein graft lesions. Eurointervention. 2008;4(1):108-114.
- Chu W, Rha SW, Kuchulakanti PK, et al. Efficacy of sirolimus-eluting stents compared with bare-metal stents for saphenous vein graft intervention. Am J Cardiol. 2006;97(1):34-37.
- Baldwin DE, Trost JC, Abbott J, et al. A comparison of drug-eluting and bare-metal stents for saphenous vein graft lesions: a report from the National Heart, Lung, and Blood Institute Dynamic Registry. J Am Coll Cardiol. 2008;51(Suppl):B23-B98.
- Vignali L, Saia F, Manari A, et al. Long-term outcomes with drug-eluting stents versus bare-metal stents in the treatment of saphenous vein graft disease (results from the REgistro Regionale AngiopLastiche Emilia-Romagna Registry). Am J Cardiol. 2008;101(7):947-952.
- Shishehbor MH, Hawi R, Singh IM, et al. Drug-eluting versus bare-metal stents for treating saphenous vein grafts. Am Heart J. 2009;158(4):637-643.
- Vermeersch P, Agostoni P, Verheye S, et al. Randomized double-blind comparison of sirolimus-eluting stent versus bare-metal stent implantation in disease saphenous vein grafts: six-month angiographic, intravascular ultrasound, and clinical follow-up of the RRISC Trial. J Am Coll Cardiol. 2006;48(12):2423-2431.
- Vermeersch P, Agostoni P, Verheye S, et al. Increased late mortality after sirolimus-eluting stents versus bare-metal stents in disease saphenous vein grafts: results from the randomized DELAYED RRISC Trial. J Am Coll Cardiol. 2007;50(3):261-267.
- Brilakis ES, Lichtenwalter C, de Lemos JA, et al. A randomized controlled trial of a paclitaxel-eluting stent versus a similar bare-metal stent in saphenous vein graft lesions the SOS (Stenting of Saphenous Vein Grafts) trial. J Am Coll Cardiol. 2009;53(11):919-928.
- Brilakis ES, Lichtenwalter C, Abdel-karim AR, et al. Continued benefit from paclitaxel-eluting compared with bare-metal stent implantation in saphenous vein graft lesions during long-term follow-up of the SOS (Stenting of Saphenous Vein Grafts) trial. JACC Cardiovasc Interv. 2011;4(2):176-182.
- Lee MS, Yang T, Kandzari DE, et al. Comparison by meta-analysis of drug-eluting stents and bare-metal stents for saphenous vein graft intervention. Am J Cardiol. 2010;105(8):1076-1082.
- Joyal D, Filion KB, Eisenberg MJ. Effectiveness and safety of drug-eluting stents in vein grafts: a meta-analysis. Am Heart J. 2010;159(2):159-169.
- Paradis JM, Belisle P, Joseph L, et al. Drug-eluting or bare-metal stents for the treatment of saphenous vein graft disease: a Bayesian meta-analysis. Circ Cardiovasc Interv. 2010;3(6):565-576.
- Lupi A, Navarese EP, Lazzero M, et al. Drug-eluting stents vs. bare-metal stents in saphenous vein graft disease. Insights from a meta-analysis of 7,090 patients. Circ J. 2011;75(2):280-289.
- Hakeem A, Helmy T, Munsif S, et al. Safety and efficacy of drug-eluting stents compared with bare-metal stents for saphenous vein graft interventions: a comprehensive meta-analysis of randomized trials and observational studies comprising 7,994 patients. Catheter Cardiovasc Interv. 2011;77(3):343-355.
- Mamas MA, Foley J, Nair S, et al. A comparison of drug-eluting stents versus bare-metal stents in saphenous vein graft PCI outcomes: a meta-analysis. J Interv Cardiol. 2011;24(2):172-180.
- Rodes-Cabau J, Bertrand OF, Larose E, et al. Comparison of plaque sealing with paclitaxel-eluting stents versus medical therapy for the treatment of moderate nonsignificant saphenous vein graft lesions: the moderate vein graft lesion stenting with the taxus stent and intravascular ultrasound (VELETI) pilot trial. Circulation. 2009;120(20):1978-1986.
- Rodes-Cabau J, Bertrand OF, Larose E, et al. Plaque sealing with paclitaxel-eluting stents for the treatment of moderate non-significant saphenous vein graft lesions. Three-year follow-up of the VELETI trial. J Am Coll Cardiol. 2010;55:A193.E1809
- Hong MK, Mehran R, Dangas G, et al. Creatine kinase-MB enzyme elevation following successful saphenous vein graft intervention is associated with late mortality. Circulation. 1999;100(24):2400-2405.
- Califf RM, Abdelmegud AE, Kuntz RE, et al. Myonecrosis after revascularization procedures. J Am Coll Cardiol. 1998;31(2):241-251.
- Kloner RA, Rude RE, Carlson N, et al. Ultrastructural evidence of microvascular damage and myocardial cell injury after coronary artery occlusion: which comes first? Circulation. 1980;62(5):945-952.
- van Gaal WJ, Banning AP. Percutaneous coronary intervention and the no-reflow phenomenon. Expert Rev Cardiovasc Ther. 2007:5(4);715-731.
- De Luca G, Suryapranata H, Stone GW, et al. Abciximab as adjunctive therapy to reperfusion in acute ST-segment elevation myocardial infarction: a meta-analysis of randomized trials. JAMA. 2005;293(14):1759-1765.
- De Luca G, Suryapranata H, Stone GW, et al. Relationship between patient’s risk profile and benefits in mortaliy from adjunctive abciximab to mechanical revascularization for ST-segment elevation myocardial infarction: a meta-regression analysis of randomized trials. J Am Coll Cardiol. 2006;47(3):685-686.
- Karha J, Gurm HS, Rajagopal V, et al. Use of platelet glycoprotein IIb/IIIa inhibitors in saphenous vein graft percutaneous coronary intervention and clinical outcomes. Am J Cardiol. 2006;98(7):906-910.
- Mathew V, Grill DE, Scott CG, et al. The influence of abciximab use on clinical outcome after aortocoronary vein graft interventions. J Am Coll Cardiol. 1999;34(4):1163-1169.
- Roffi M, Mukherjee D, Chew DP, et al. Lack of benefit from intravenous platelet glycoprotein IIb/IIIa receptor inhibition as adjunctive treatment for percutaneous interventions of aortocoronary bypass grafts: a pooled analysis of five randomized clinical trials. Circulation. 2002;106(24):3063-3067.
- Mak KH, Challapalli R, Eisenberg MJ, et al. Effect of platelet glycoprotein IIb/IIIa receptor inhibition on distal embolization during percutaneous revascularization of aortocoronary saphenous vein grafts. EPIC Investigators. Evaluation of IIb/IIIa platelet receptor antagonist 7E3 in Preventing Ischemic Complications. Am J Cardiol. 1997;80(8):985-988.
- Jonas M, Stone GW, Mehran R, et al. Platelet glycoprotein IIb/IIIa receptor inhibitos as adjunctive treatment during saphenous vein graft stenting: differential effects after randomization to occlusion or filter-based embolic protection. Eur Heart J. 2006;27(8):920-928.
- Lee CH, Low A, Tai BC, et al. Pretreatment with intracoronary adenosine reduces the incidence of myonecrosis after non-urgent percutaneous coronary intervention: a prospective randomized study. Eur Heart J. 2007;28(1):19-25.
- Desmet WJ, Dens J, Coussement P, Van de Werf F. Does adenosine prevent myocardial micronecrosis following percutaneous coronary intervention? The ADELINE pilot trial. ADEnosine LImit myocardial Necrosis. Heart. 2002;88(3):293-295.
- Marzilli M, Orsini E, Marraccini P, Testa R. Beneficial effects of intracoronary adenosine as an adjunct to primary angioplasty in acute myocardial infarction. Circulation. 2000;101(18):2154-2159.
- Stoel MG, Marques KM, de Cock CC, et al. High dose adenosine for suboptimal myocardial perfusion ater primary PCI: a randomized placebo-controlled pilot study. Catheter Cardiovasc Interv. 2008;71(3):283–289.
- Assali AR, Sdringola S, Ghani M, et al. Intracoronary adenosine administered during percutaneous intervention in acute myocardial infarction and reduction in the incidence of “no reflow” phenomenon. Catheter Cardiovasc Interv. 2000;51(1):27-32.
- Magaffey KW, Puma JA, Barbagelata NA, et al. Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: results of a multicenter, randomized placebo-controlled trial: the Acute Myocardial Infarction STudy of ADenosine (AMISTAD) trial. J Am Coll Cardiol. 1999;34(6):1711-1720.
- Kloner RA, Forman MB, Gibbons RJ, Ross AM, Alexander RW, Stone GW. Impact of time to therapy and reperfusion modality on the efficacy of adenosine in acute myocardial infarction: the AMISTAD-2 trial. Eur Heart J. 2006;27(20):2376-2377.
- Fischell TA, Carter AJ, Foster MT, et al. Reversal of “no reflow” during vein graft stenting using high velocity boluses of intracoronary adenosine. Cathet Cardiovasc Diagn. 1998;45(4):360-365.
- Sdringola S, Assali A, Ghani M, et al. Adenosine use during aortocornary vein graft interventions reverses but does not prevent the slow-no reflow phenomenon. Catheter Cardiovasc Interv. 2000;51(4):394-399.
- Watts JA, Hawes EM, Jenkins SH, et al. Effects of nisoldipine on the no-reflow phenomenon in globally ischemic rat hearts. J Cardiovasc Pharmacol. 1990:16(3):487-494.
- Villari B, Ambrosio G, Gollino P, et al. The effects of calcium channel antagonist treatment and oxygen radical scavenging on infarct size and the no-reflow phenomenon in reperfused hearts. Am Heart J. 1993;125(1):11-23.
- Taniyama Y, Ito H, Iwakura K, et al. Beneficial effect of intracoronary verapamil on microvascular and myocardial salvage in patients with acute myocardial infarction. J Am Coll Cardiol. 1997:30(5):1193-1199.
- Fugit MD, Rubal BJ, Donovan DJ. Effects of intracoronary nicardipine, diltiazem and verapamil on coronary blood flow. J Invasive Cardiol. 2000:12(2):80-85.
- Vijayalakshmi K, Whittaker VJ, Kunadian B, et al. Prospective, randomized, controlled trial to study the effect of intracoronary injection of verapamil and adenosine on coronary blood flow during percutaneous coronary intervention in patients with acute coronary syndromes. Heart. 2006:92(9):1278-1284.
- Michaels AD, Appleby M, Otten MH, et al. Pretreatment with intragraft verapamil prior to percutaneous coronary intervention of saphenous vein graft lesions: results of the randomized, controlled vasodilator prevention on no-reflow (VAPOR) trial. J Invasive Cardiol. 2002;14(6):299-302.
- Kaplan BM, Benzuly KH, Kinn JW, et al. Treatment of no-reflow in degenerate saphenous vein graft interventions: comparison of intracoronary verapamil and nitroglycerin. Cathet Cardiovasc Diagn. 1996;39(2):113-118
- Piana RN, Paik GY, Mosucci M, et al. Incidence and treatment of “no re-flow” after percutaneous coronary intervention. Circulation. 1994;89(6):2514-2518.
- Resnic FS, Wainstein M, Lee MK, et al. No-reflow is an independent predictor of death and myocardial infarction after percutaneous coronary intervention. Am Heart J. 2003;145(1):42-46.
- Huang RI, Patel P, Walinsky, P, et al. Efficacy of intracoronary nicardipine in the treatment of no-reflow during percutaneous coronary intervention. Catheter Cardiovasc Interv. 2006;68(5):671-676.
- Fischell TA, Subraya RG, Ashraf K, et al. “Pharmacologic” distal protection using prophylactic, intragraft nicardipine to prevent no-reflow and non-Q wave myocardial infarction during elective saphenous vein graft intervention. J Invasive Cardiol. 2007;19(2):58-62.
- Abbo KM, Dooris M, Glazier S, etal. Features and outcome of no-reflow after percutaneous coronary intervention. Am J Cardiol. 1995;75(12):778-782.
- Baldus S, Koster R, Elsner M, et al. Treatment of aortocoronary vein graft lesions with membrane-covered stents: a multicenter surveillance trial. Circulation. 2000;102(17):2024-2027.
- Schachinger V, Hamm CW, Munzel T, et al; STING (STents IN Grafts) Investigators. A randomized trial of polytetrafluoroethylene-membrane-covered stents compared with conventional stents in aortocoronary saphenous vein grafts. J Am Coll Cardiol. 2003;42(8):1360-1369.
- Stankovic G, Colombo A, Presbitero P, et al. Randomized Evaluation of polytetrafluoroethylene COVERed stent in Saphenous vein grafts (RECOVERS) Trial. Circulation. 2003;108(1):37-42.
- Turco MA, Buchbinder M, Popma JJ, et al. Pivotal, randomized U.S. study of the SymbiotTM covered stent system in patients with saphenous vein graft disease: eight-month angiographic and clinical results from the Symbiot III trial. Catheter Cardiovasc Interv. 2006;68(3):379-388.
- Stone GW, Goldberg S, O’Shaughnessy C, et al. 5-year follow-up of polytetrafluoroethylene-covered stents compared with bare-metal stents in aortocoronary saphenous vein grafts the randomized BARRICADE (barrier approach to restenosis: restrict intima to curtail adverse events) trial. JACC Cardiovasc Interv. 2011;4(3):300-309.
- Baim DS, Wahr D, George B, et al. Randomized trial of a distal embolic protection device during percutaneous intervention of saphenous vein aortocoronary bypass grafts. Circulation. 2002;105(11):1285-1290.
- Carrozza Jr. JP, Mumma M, Breall JA, et al; the PRIDE Study Investigators. Randomized Evaluation of the TriActiv Balloon-Protection Flush and Extraction System for the Treatment of Saphenous Vein Graft Disease. J Am Coll Cardiol. 2005;46(9):1677-1683.
- Stone G, Rogers C, Hermiller J, et al. Randomized comparison of distal protection with a filter-based catheter and a balloon occlusion and aspiration system during percutaneous intervention of diseased saphenous vein aorto-coronary bypass grafts. Circulation. 2003;108(5):548-553.
- Cox DA. Stenting in saphenous vein grafts with distal protection using a second generation filter-based catheter: the combined BLAZE I and II registries. Paper presented at: Transcatheter Cardiovascular Therapeutics; Oct 2005; Washington, DC.
- Dixon SR. Saphenous vein graft protection in a distal embolic protection randomized trial. Paper presented at: Transcatheter Cardiovascular Therapeutics; Oct 2005; Washington, DC.
- Kereiakes DJ, Turco MA, Breall J, et al. A novel filter-based distal embolic protection device for percutaneous intervention of saphenous vein graft lesions: results of the AMEthyst randomized controlled trial. JACC Cardiovasc Interv. 2008;1(3):248-257.
- Mauri L, Cox DA, Hermiller J, et al. The PROXIMAL trial: proximal protection during saphenous vein graft intervention using the Proxis embolic protection system: a randomized, prospective, multicenter trial. J Am Coll Cardiol. 2007;50(15):1142-1449.
- Smith Jr SC, Feldman TE, Hirshfeld Jr JW, et al. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2006;113(7):E166-E286.
- Mehta SK, Frutkin AD, Milford-Beland S, et al. Utilization of distal embolic protection in saphenous vein graft interventions (an analysis of 19,546 patients in the American College of Cardiology-National Cardiovascular Data Registry). Am J Cardiol. 2007;100:1114–1118.
- Kohsaka S, Makaryus AN. Coronary angiography using noninvasive imaging techniques of cardiac CT and MRI. Curr Cardiol Rev. 2008;4(4):323-330.
- Schlosser T, Konorza T, Hunold P, et al. Noninvasive visualization of coronary artery bypass grafts using 16-detetor row computed tomography. J Am Coll Cardiol. 2004;44(6):1224-1229.
- Safian RD, Freed MS, eds. The Manual of Interventional Cardiology. Royal Oak, MI: Physicians Press, 2001.
- Collaborative overview of randomized trials of antiplatelet therapy — II: maintenance of vascular graft or arterial patency by antiplatelet therapy. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308(6922):159-168.
- Gavaghan TP, Gebski V, Baron DW. Immediate postoperative aspirin improves vein graft patency early and late after coronary artery bypass graft surgery. A placebo-controlled, randomized study. Circulation. 1991;83(5):1526-1533.
- The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. The Post Coronary Artery Bypass Graft Trial Investigators. N Engl J Med. 1997;336(3):153-162. Erratum in: N Engl J Med. 1997;337(25):1859.
- Domanski MJ, Borkowf CB, Campeau L, et al. Prognostic factors for atherosclerosis progression in saphenous vein grafts: post-CABG trial. J Am Coll Cardiol. 2000;36(6):1877-1883.
_____________________________________
From the Department of Cardiovascular Medicine, Hahnemann University Hospital, Philadelphia, Pennsylvania.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted September 15, 2011, provisional acceptance given October 11, 2011, final version accepted November 15, 2011.
Address for correspondence: Sheldon Goldberg, MD, Hahnemann University Hospital, Broad & Vine Streets, 7th Floor South Tower, Philadelphia, PA 19102. Email: goldbergshel@yahoo.com
















