The Role of Intravascular Optical Coherence Tomography in Peripheral Percutaneous Interventions
Download a PDF of this article.
Abstract: Frequency domain optical coherence tomography (FD-OCT) is a new intravascular imaging modality utilizing near-infrared light to generate high-quality images. It provides a unique and detailed view of the vessel wall and vessel lumen. FD-OCT has been increasingly used in coronary interventions; however, there is limited experience regarding the use of FD-OCT in the evaluation and treatment of peripheral arterial disease (PAD). We report three cases in which FD-OCT helped elucidate and guide femoral artery interventions and renal artery stenting. With the help of these cases, we depict how FD-OCT is uniquely positioned to be a promising imaging tool in the diagnosis and treatment of peripheral artery disease.
J INVASIVE CARDIOL 2013;25(3):E51-E53
Key words: optical coherence tomography, peripheral vascular disease, percutaneous intervention
__________________________________________
Optical coherence tomography (OCT) is a novel and rapidly evolving intravascular imaging technique.1 It was first described by Huang et al in 1991, with its first application being as an ocular imaging modality.2 OCT has since become an established clinical imaging technique in the fields of ophthalmology, gastroenterology, dermatology, and more recently, interventional cardiology. An intracoronary, commercially-available system is now approved for use in the United States. OCT uses near-infrared light to generate images in the order of 10 micron resolution.1 OCT image acquisition time is short, which is ideal for an intravascular imaging tool.1
Intravascular ultrasound (IVUS) imaging technique utilizes sound waves to generate images. OCT has certain advantages over IVUS. The resolution of IVUS is 100 microns, whereas that of OCT is in the range of 10 microns,3 making its resolution superior.3 Current intravascular OCT catheters image at 100 frames/s, corresponding to 6000 rotations per minute. IVUS, on the other hand, has a frame rate of only 30 frames/s. A higher frame rate corresponds to an ability to image longer segments of the artery and shortens the procedure time.3 Another advantage of OCT is its faster pull-back rate of 20 mm/sec, in contrast to an IVUS pull-back rate of only 0.5-1 mm/sec. However, OCT also has its disadvantages when compared with IVUS. Tissue penetration of IVUS is 10 mm, compared to only 0.5-2.0 mm for OCT. In addition, OCT is limited by the maximal vessel size, making it less useful in imaging ostial lesions and those in the left main coronary artery.
Over the last few years, there has been a surge in the use of OCT in coronary diagnostic and interventional procedures, including complex coronary interventions.4-7 With its ultra-high resolution properties, OCT provides a detailed analysis of coronary stent strut apposition, neointimal formation, stent thrombus, dissection flaps, as well as unique in vivo plaque characterization.1,6,7
Blood strongly scatters light and, therefore, creating a blood-free field lasting several seconds is crucial to successful image acquisition by intravascular OCT. The coronary vessels can be rendered free of blood by a 12-15 cc bolus injection of contrast injected at a rate of 2-4 mL/sec. Complete or near-complete opacification with contrast is needed for an optimal imaging field. In peripheral cases, the sheath must be in close proximity to the site of interest. The standard OCT catheter is compatible with a 6 (French) Fr sheath.3
Second-generation frequency domain OCT (FD-OCT) provides imaging of the coronary lumen with a fast and automated pullback, 20 mm/sec.8 It has the potential to improve outcomes in percutaneous interventions by providing a clearer visualization of the vessel wall and of the applied treatment.
To date, minimal clinical experience is available on the use of FD-OCT in the peripheral vessels. With the following three case illustrations, we introduce and depict potential new applications for FD-OCT in peripheral vascular interventions.
Case Descriptions
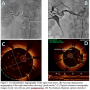 Case 1. A 73-year-old Caucasian male presented to the hospital with chest pain, headache, new-onset hypertension, and radiographic evidence of pulmonary edema. His presenting blood pressure was 210/110 mm Hg. A renal arterial ultrasound as well as a computed tomography (CT) renal angiogram suggested the presence of right renal artery stenosis.
Case 1. A 73-year-old Caucasian male presented to the hospital with chest pain, headache, new-onset hypertension, and radiographic evidence of pulmonary edema. His presenting blood pressure was 210/110 mm Hg. A renal arterial ultrasound as well as a computed tomography (CT) renal angiogram suggested the presence of right renal artery stenosis.
Interventional procedure. A 7 Fr renal guide was used to cannulate the right renal artery. Quantitative angiography depicted a 4 mm right renal artery diameter with >80% ostial stenosis (Figure 1A). The lesion was crossed with a 0.014˝ High-Torque Floppy II ES guidewire (Abbott Vascular) and primarily stented with a 4 x 15 mm Palmaz Blue stent (Cordis Corporation). Post stent deployment angiography demonstrated an excellent angiographic result (Figure 1B). A C7 Dragonfly FD-OCT catheter (St Jude Medical) was then placed in the mid-right renal artery and images were acquired. Images revealed a 5 mm vessel diameter with very obvious stent malapposition (Figure 1C). Postdilation with a 5 x 12 mm NC Quantum Apex (Boston Scientific) was then performed. Repeat FD-OCT images showed improved stent apposition and a >15 mm2 vessel area (Figure 1D).
 Case 2. A 77-year-old African-American woman with a history of hypertension, diabetes mellitus, dyslipidemia, coronary heart disease, and peripheral artery disease (PAD) presented with resting pain in her right foot. She had undergone right superficial femoral artery (SFA) percutaneous transluminal angioplasty (PTA) and stent placement 12 months earlier. Physical exam revealed absent pulses in her right foot.
Case 2. A 77-year-old African-American woman with a history of hypertension, diabetes mellitus, dyslipidemia, coronary heart disease, and peripheral artery disease (PAD) presented with resting pain in her right foot. She had undergone right superficial femoral artery (SFA) percutaneous transluminal angioplasty (PTA) and stent placement 12 months earlier. Physical exam revealed absent pulses in her right foot.
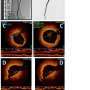
Interventional procedure. A 65 cm, 6 Fr sheath was advanced into the proximal right SFA using the up-over technique. Angiography revealed distal SFA in-stent restenosis with apparent stent fracture (Figure 2A). Sequential PTA of the right SFA was performed with 4 mm and 5 mm Power-flex balloons (Cordis Corporation). The dilatation with the 5 mm balloon caused a localized SFA perforation, which was contained with low-pressure balloon inflations. Visual angiographic residual stenosis was estimated at 30% by several operators (Figure 2B). A C7 Dragonfly catheter was then used to image the vessel and stent. FD-OCT images were acquired before and after intervention. FD-OCT revealed a residual stenosis of 60% (reference area, 25.72 mm2; target area, 8.6 mm2; Figure 2C). Stent fracture is depicted in Figure 2D.
 Case 3. A 77-year-old African-American woman with diabetes mellitus, hypertension, coronary heart disease, and progressive lifestyle-limiting claudication of the left lower extremity was referred for angiography of the left lower extremity. Left foot pulses were not palpable.
Case 3. A 77-year-old African-American woman with diabetes mellitus, hypertension, coronary heart disease, and progressive lifestyle-limiting claudication of the left lower extremity was referred for angiography of the left lower extremity. Left foot pulses were not palpable.
Interventional procedure. A 75 cm, 7 Fr sheath was placed in the right common femoral artery and then advanced to the left SFA using the up-over technique. Left SFA angiography revealed a heavily calcified and stenotic vessel with 1 vessel run-off (peroneal artery). A Whisper 0.014˝ guidewire (Abbott Vascular) was advanced to the distal left peroneal artery. After the left SFA lesion was predilated with a 2.5 x 40 mm Sleek balloon (Cordis Corporation), a 4 mm SpiderFX embolic device (eV3) was placed in the mid-left peroneal artery. FD-OCT images with the C7 Dragonfly catheter were obtained before and after excisional atherectomy (TurboHawk SX-C; eV3) and balloon angioplasty with a 4 x 120 mm Sleek balloon inflated up to 6 atm. A 6 x 120 mm Life Science stent (CR Bard) was then deployed in the SFA with excellent angiographic results (Figures 3A, 3B, 3C, and 3D).
Discussion
The three cases described above illustrate some potential applications of FD-OCT in peripheral vessels. The ultra-high resolution images of the vessel lumen, stents, and the vessel wall provided mechanistic insights for treatment failure in patients receiving renal stents guided by quantitative angiography only, in restenotic SFA lesions treated with plain balloon angioplasty, and helped illustrate the limitations and potential dangers of “blind cutting” with excisional atherectomy.
There are several learning points in these cases. In case #1, FD-OCT revealed an obvious right renal stent malapposition in a patient with an “optimal angiographic result.” Stent malapposition is the lack of contact between stent struts and the underlying vessel wall9,10 and is a well-known risk factor for stent thrombosis and restenosis.11,12 Due to its higher resolution, FD-OCT visualizes the degree of malapposition of stent struts in greater detail than IVUS. Renal stent underexpansion with its subsequent incomplete strut apposition is of great interest, since it is to some extent avoidable. A better and more accurate deployment technique in renal vessels may lead to lower short- and long-term failure rates in renovascular interventions. In this case, FD-OCT images led to appropriate balloon size selection with a final excellent stent expansion and apposition.
Case #2 illustrates how FD-OCT helped define the “true % residual stenosis” following balloon angioplasty of a heavily restenotic SFA segment in a patient with an apparent “good visual angiographic result” of <30%. FD-OCT residual stenosis was close to 60%. This observation may help explain the high failure rate of restenotic SFA lesions treated with plain old balloon angiplasty only. Better patient outcome may be achieved with more reliable data provided by high-resolution intravascular imaging as with FD-OCT.
Case #3 illustrates the use of FD-OCT imaging in a patient treated with excisional atherectomy. This approved technique is used to modify fibrocalcific lesions prior to balloon angioplasty with or without stent implantation. The TurboHawk uses a cutting blade that is manipulated under fluoroscopic guidance. In this case, FD-OCT depicts the therapeutic pitfalls and potential dangers of “blind cutting” of plaques. Unequal plaque removal is clearly demonstrated by FD-OCT in this case (Figures 3A, 3B, 3C, and 3D). The excessive excision of plaque and of the vessel wall in some segments nearly reached the adventitial layer, whereas plaque removal was clearly suboptimal in several other areas. New atherectomy devices with incorporated IVUS or OCT imaging capabilities could lead to better outcomes and fewer complications, ie, dissections, perforations, and vessel closures.
In summary, FD-OCT has the potential of becoming a very useful technique in peripheral vascular interventions. Its unique ultra-high resolution imaging capability is a welcome addition to our vascular tool box.
References
- Kawasaki M, Takatsu H, Noda T, et al. In vivo quantitative tissue characterization of human coronary arterial plaques by use of integrated backscatter intravascular ultrasound and comparison with angioscopic findings. Circulation. 2002;105(21):2487-2492.
- Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254(5035):1178-1181.
- Lowe HC, Narula J, Fujimoto JG, Jang IK. Intracoronary optical diagnostics current status, limitations, and potential. JACC Cardiovasc Interv. 2011;4(12):1257-1270.
- Yock PG, Linker DT. Intravascular ultrasound Looking below the surface of vascular disease. Circulation. 1990;81(5):1575–1585.
- Yamaguchi T, Terashima M, Akasaka T, et al. Safety and feasibility of an intravascular optical coherence tomography image wire system in the clinical setting. Am J Cardiol. 2008;101(5):562-567.
- Matsumoto D, Shite J, Shinke T, et al. Neointimal coverage of sirolimus-eluting stents at 6-month follow-up: evaluated by optical coherence tomography. Eur Heart J. 2007;28(8):961-967.
- Sawada T, Shite J, Shinke T, et al. Persistent malapposition after implantation of sirolimus-eluting stent into intramural coronary hematoma: optical coherence tomography observations. Circ J. 2006;70(11):1515-1519.
- Okamura T, Gonzalo N, Gutierrez-Chico JL, et al. Does the second generation OCT improve safety and feasibility in clinical practice? A single center experience. J Am Coll Cardiol. 2010;55:E1906.
- Finn AV, Joner M, Nakazawa G, et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115(18):2435–2441.
- Cook S, Wenaweser P, Togni M, et al. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation. 2007;115(18):2426–2434.
- Guagliumi G, Sirbu V, Musumeci G, et al. Examination of the in vivo mechanisms of late drug-eluting stent thrombosis. JACC Cardiovasc Interv. 2012;5(1):12–20.
- Ozaki Y, Okumura M, Ismail TF, et al. The fate of incomplete stent apposition with drug-eluting stents: an optical coherence tomography based natural history study. Eur Heart J. 2010;31(12):1470–1476.
__________________________________________
From the University of Texas Health Science Center, Houston, Texas.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted September 4, 2012, provisional acceptance given September 12, 2012, final version accepted September 20, 2012.
Address for correspondence: Oscar Rosales, MD, FACC, FSCAI, Medical Director, Cardiac Catheterization Laboratories, Memorial Hermann Hospital, Texas Medical Center, Division of Cardiology, University of Texas at Houston, Houston, TX 77030. Email:orosales@houstoncardiovascular.com


















