Practical Considerations of Percutaneous Ventricular Assist Devices
Abstract: Percutaneous ventricular assist devices are increasingly used today, allowing the scope of left ventricular support to move out of the operating room and into the interventional suite and cardiovascular care unit. This has given patients requiring short-term therapy an opportunity to undergo high-risk procedures and provide a failing left ventricular support until native recovery can occur. A growing body of evidence exists that demonstrates device efficacy and safety, as well as its potential clinical importance, a topic that will be reviewed here. Additionally, many providers seek a resource for technical considerations and troubleshooting. We also aim to provide insight into such considerations.
J INVASIVE CARDIOL 2012;24(1):25-31
Key words: percutaneous left ventricular support, percutaneous left ventricular assist device, cardiogenic shock, high-risk percutaneous coronary intervention
_______________________________________
Historical Perspective
The concept of mechanical circulatory support has matured over several decades, with the first long-term surgical application in humans in 1966.1 The original design of surgical ventricular assist devices (VADs) attempted to mimic cardiopulmonary bypass, and allowed patient’s passage to hospital units where continued myocardial recovery could be supported at the bedside via large, yet moveable consoles. The subsequent growing need for long-term support in the era of cardiac transplantation, where many patients died awaiting heart donation, fueled the development of more sophisticated intracorporeal devices that not only allowed movement out of the operating room, but also movement out of the hospital. Yet many patients require effective circulatory support for short or intermediate duration where surgical VADs are not warranted, making temporary percutaneous options feasible and necessary. Until recently, the only option available for such indications was the intra-aortic balloon pump (IABP).
The tenet of aortic counterpulsation was first described in 1962 by Moulopoulos, using sequenced inflation and deflation of a catheter-mounted polyethylene balloon in the descending aorta.2 Its first clinical use in two subjects with cardiogenic shock in 1968 demonstrated improvement in systemic arterial and central venous pressures, but required a femoral cutdown.3 In 1980, Bregman described percutaneous insertion,4 with the most recent iterations achieving 7.5 Fr sizing. Ideally placed 1-2 cm below the origin of the left subclavian artery and above the renal arteries, rapid balloon inflation during diastole augments diastolic pressure, thereby increasing driving pressure for coronary perfusion. Aortic volume, and thus afterload, is reduced via a vacuum effect during rapid balloon deflation in systole. These effects can be quite variable, depending on the volume of the balloon, balloon position in the aorta, systemic resistance, and aortic compliance,5 but the majority of patients generally see a decrease in systolic pressure by 20 mm Hg, an increase in aortic diastolic pressure by 30%, an increase in mean arterial pressure (MAP), a reduction in heart rate and pulmonary capillary wedge pressure (PCWP) by 20%, and an increase in cardiac output by 20%.6 Despite its commonplace use in coronary care units and interventional suites, however, patients with ischemic or non-ischemic cardiomyopathy and cardiogenic shock supported with IABPs continue to see mortality rates of 55% to 73%.7 Additionally, recent data have found that the use of IABP during high-risk percutaneous coronary interventions (PCI) in patient with severe left ventricular (LV) dysfunction did not result in a reduction in major adverse cardiovascular events, remaining high at 15-16%.8 Evidence thus suggested that higher-level support was necessary in certain clinical scenarios, giving way to the development of active percutaneous circulatory support options. Impella 2.5 (Abiomed) and TandemHeart (Cardiac Assist, Inc.) are two such devices that are currently available for use in the United States. Unlike IABP, which supports cardiac function in a passive manner, they are increasingly used to actively support high-risk coronary interventions as well as cardiac output in the acutely failing ventricle.
Principles of Hemodynamic Support
In the modern era, the ideal percutaneous mechanical support device should meet two basic tenets. First, it should provide ease of insertion (such as the IABP) and second, it should augment forward flow in an active manner (unlike the IABP, which indirectly augments cardiac output by reducing afterload). Yet, it is often true that one goal is at the expense of the other. Pumps that provide the most augmentation in forward flow require larger cannulae and pumping chambers by design, ultimately impeding the ease of percutaneous insertion. Those that allow for streamlined access are limited in the volume of blood that can be mobilized per unit of time. It is important to stress, however, that forward flow is vital and should meet a certain volume threshold because of the important secondary functions this provides. First, improved forward flow unloads the ventricle by reducing LV end-diastolic filling pressures and volume, thereby decreasing wall tension and myocardial oxygen demand. Second, reduced filling parameters improve circulation through the microvasculature, allowing for increased perfusion pressure and myocardial oxygen supply. This volume threshold is currently undefined, but likely exists in the range of 2-3 L/min based on anecdotal experience.
 In the absence of a better investigative tool, LV hemodynamic parameters are often characterized by pressure-volume (PV) loops depicting the changes in ventricular pressure and volume during a complete cardiac cycle (Figure 1). Point 1 represents end-diastolic pressure and volume prior to isovolemic contraction, at which point pressure rises quickly until it exceeds aortic pressure and the aortic valve opens at point 2. The pressure climbs and then falls throughout systole until point 3, when the LV pressure falls below the aortic pressure and the aortic valve again closes, representing the end of systole. The pressures continue to fall during isovolemic relaxation to point 4, which represents the start of diastole. The volume change between points 2 and 3 represents stroke volume, and the area within the loop represents the stroke work performed by the ventricle during each cardiac cycle. The loop is bound by the end-diastolic pressure-volume relationship (EDPVR) inferiorly, representing passive filling properties of the LV, and by the end-systolic pressure-volume relationship (ESPVR) superiorly, loosely representing LV contractility.
In the absence of a better investigative tool, LV hemodynamic parameters are often characterized by pressure-volume (PV) loops depicting the changes in ventricular pressure and volume during a complete cardiac cycle (Figure 1). Point 1 represents end-diastolic pressure and volume prior to isovolemic contraction, at which point pressure rises quickly until it exceeds aortic pressure and the aortic valve opens at point 2. The pressure climbs and then falls throughout systole until point 3, when the LV pressure falls below the aortic pressure and the aortic valve again closes, representing the end of systole. The pressures continue to fall during isovolemic relaxation to point 4, which represents the start of diastole. The volume change between points 2 and 3 represents stroke volume, and the area within the loop represents the stroke work performed by the ventricle during each cardiac cycle. The loop is bound by the end-diastolic pressure-volume relationship (EDPVR) inferiorly, representing passive filling properties of the LV, and by the end-systolic pressure-volume relationship (ESPVR) superiorly, loosely representing LV contractility.
Ultimately, the purpose of this model is to provide information about myocardial oxygen consumption, which is most accurately represented by the pressure-volume area (PVA). The PVA is the area bound by the ESPVR, the EDPVR, and the systolic component of the PV loop. Represented here are: (1) the area within the loop correlating to stroke work (mechanical energy); (2) the wall tension (potential energy) stored at end-diastole; and (3) the area to the left of the loop correlating to the residual potential energy stored by stretched myocardium at the end of each beat. A model of ventricular unloading would therefore: (1) decrease the potential energy of LV end-diastolic and end-systolic volumes (shifting points 1 and 3 to the left); and (2) reduce stroke work (change between points 2 and 3). This is a simplified model, however, which assumes that the contractile state of the myocardium (represented by the ESPVR) remains unchanged and all unloading is accomplished by the above-mentioned mechanisms. The actual mechanics of LV unloading do likely involve changes to myocardial contractility, but are beyond the scope of this review.
Impella 2.5 and TandemHeart provide active cardiac unloading by diverting blood flow away from the LV and directly into the aorta. Although unique in the mechanism by which they accomplish this goal, the basic principles described above are met by both systems.
The Mechanics Behind Percutaneous Support
The parent design of percutaneous LVADs is pulsatile, operating by the positive displacement of blood, mimicking normal cardiac mechanics. An externalized pumping chamber propels blood from an upstream compartment (ie, the LV) via an inlet cannula to a downstream compartment (ie, descending aorta) via an outlet cannula. The overall effect, and the concept all VADs attempt to replicate, is diversion of blood from the failing ventricle. However, pulsatile devices are sizable and patient habitus limit their use, thus fueling the development of significantly smaller continuous flow pumps which allow for percutaneous insertion.9
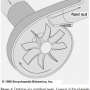 Continuous pumps exist in two varieties, centrifugal and axial, each operating with unique flow dynamics. Centrifugal pump design includes a rotating component with one or more impeller vanes, which transfer energy derived from a motor to the blood being pumped and accelerate blood outward away from the center of rotation in a “centrifugal” manner (Figure 2).10 The TandemHeart functions via these mechanics.
Continuous pumps exist in two varieties, centrifugal and axial, each operating with unique flow dynamics. Centrifugal pump design includes a rotating component with one or more impeller vanes, which transfer energy derived from a motor to the blood being pumped and accelerate blood outward away from the center of rotation in a “centrifugal” manner (Figure 2).10 The TandemHeart functions via these mechanics.
Axial flow pumps, like Impella 2.5, also consist of rotating impellers in a pump housing, but unlike centrifugal pumps which situate inlet and outlet cannulae perpendicularly, blood flow here is aligned longitudinally. Simply, the rotating impeller vanes create a negative pressure differential, or vacuum, which suctions blood from the inlet, through the pumping unit, and to the device outlet.
 The specific function of the axial flow pump depends on several mechanical and electrical factors. First, the speed at which the impeller vanes spin depends in part on the voltage delivered to the pump motor, which in turn is set and altered via the device console. Higher voltage translates into increased rotations per minute. Second, resistance in the pump circuit is affected by loading conditions. Simply, circuit resistance is minimized when the pressure difference across the pump inlet and outlet chambers is small. The device current (which closely approximates blood flow) is dependent on both of these factors: voltage supplied and circuit resistance. Since voltage is constant and resistance changes during systole and diastole, the resultant device current is dynamic and changes throughout the cardiac cycle. As such, some degree of pulsatility may be generated given native left ventricular contractility and the change in resistance this creates (Figure 3). For example, during cardiac systole when LV pressure approximates aortic pressure, the resistance in the circuit is minimized, thereby increasing pump speed and blood flow compared to diastole. Similarly, higher LV diastolic pressures reduce the pressure difference between aorta and LV, again increasing pump speed and blood flow.
The specific function of the axial flow pump depends on several mechanical and electrical factors. First, the speed at which the impeller vanes spin depends in part on the voltage delivered to the pump motor, which in turn is set and altered via the device console. Higher voltage translates into increased rotations per minute. Second, resistance in the pump circuit is affected by loading conditions. Simply, circuit resistance is minimized when the pressure difference across the pump inlet and outlet chambers is small. The device current (which closely approximates blood flow) is dependent on both of these factors: voltage supplied and circuit resistance. Since voltage is constant and resistance changes during systole and diastole, the resultant device current is dynamic and changes throughout the cardiac cycle. As such, some degree of pulsatility may be generated given native left ventricular contractility and the change in resistance this creates (Figure 3). For example, during cardiac systole when LV pressure approximates aortic pressure, the resistance in the circuit is minimized, thereby increasing pump speed and blood flow compared to diastole. Similarly, higher LV diastolic pressures reduce the pressure difference between aorta and LV, again increasing pump speed and blood flow.
Impella 2.5
Technical considerations. The Impella 2.5 is a catheter-mounted microaxial flow pump placed via the femoral artery which expels blood directly from the left ventricle into the ascending aorta, providing up to 2.5 liters per minute of flow. When properly placed, the blood inlet is situated in the LV outflow tract approximately 4 cm below the aortic valve and the blood outlet several centimeters above the aortic cusps. The Impella 2.5 catheter is introduced via a 13 Fr femoral arterial sheath that is advanced over a 0.018˝ guidewire in a monorail fashion, and is placed across the aortic valve with guidance of fluoroscopy as well as a pressure sensing mechanism that displays the position of the device on the Impella console. Echocardiography has also been shown to effectively assist in device placement.11 In the authors’ experience, the device can be advanced without the assistance of a guidewire. Once at the aortic cusps, setting the pump speed at P2 assists in suctioning the pigtail catheter across the aortic valve and into the LV using a standard prolapsing technique. Once in place, the device is started at its minimum setting (P1 or P2) to offset catheter-induced aortic regurgitation, at which point performance levels are increased to meet the hemodynamic needs of the patient. Pump flows displayed on the console are derived numbers based on the current flowing through the system as actual flows are never directly measured. Additionally, although P9 provides more flow than P8, the incremental flow achieved by increasing from P8 to P9 is not as great as the change from say P3 to P4 for a majority of loading conditions encountered in a standard cardiac patient. Generally, a current of 0.5-0.6 mAmp represents a well-functioning system. Heparin is used while the Impella remains in place, with a target activated clotting time (ACT) of 250 seconds. The United States Food and Drug Administration (FDA) currently approves the device under 510(k) clearance for partial circulatory support for up to 6 hours, with clinical trials allowing use up to 7 days in the United States and 10 days in Europe.
If positioning alarms sound, the pump may be too far forward with both inlet and outlet areas positioned in the LV, or too far back with inlet and outlet in the aorta. If too far in the LV, a pullback until an aortic waveform is visible on the console can be performed. If too far into the aorta, an attempt at re-crossing the aortic valve can be made, but may require fluoroscopic guidance. In the authors’ experience, the blood outlet can be easily identified by the position of the color mosaic generated on Doppler echocardiogram, ideally beyond the aortic cusps.11 Further, extended pump usage occasionally results in purge pressure alarms. A 20% dextrose solution is used to purge the catheter at the level of the motor housing in a retrograde manner, thereby deflecting blood away from the motor. One solution to such alarms is to decrease the viscosity of the purging fluid by changing to 10% or 5% dextrose solution. However, these alarms could also be secondary to a mechanical disruption of the catheter or kinking that artificially elevates purge pressure. In this instance, the motor current would either remain the same or even increase, but the motor may not be spinning effectively. Color Doppler examination may be useful to confirm motor function, as evidenced by the previously described color mosaic.
Absolute contraindications for use of the Impella device include the presence of a mechanical aortic valve, severe aortic stenosis or regurgitation, and presence of an LV thrombus. However, a significant proportion of Impella-related complications are that of limb ischemia secondary to severe peripheral vascular disease. Therefore, this relative contraindication of severe peripheral vascular disease is of great clinical importance. However, several studies have demonstrated the acceptable safety profile of Impella use in human subjects.12-17 Despite concerns for hemolysis, bleeding with need for transfusion, and damage to the aortic valve, these complications occur infrequently. On the contrary, if device usage extends beyond FDA-labeling, limb ischemia and ineffective pump function because of pump position changes may become more relevant, making monitoring for limb viability, position of the device, and maintenance of adequate anticoagulation critical.
Outcomes data. The earliest studies evaluating the effect of Impella on cardiac hemodynamics involved the surgically-placed Impella 5.0 device in both animals and humans. In cardiogenic shock, Impella 5.0 demonstrated a significant improvement in cardiac output,18,19 MAP,18-20 and coronary blood flow.19,21 These studies additionally demonstrated significant LV unloading with reductions in LV end-diastolic pressure (LVEDP), left atrial pressure, and PCWP.18-21 This translated into improvements in myocardial oxygen consumption, decreased lactate production, and decreased LV work.
Contemporary studies evaluating the Impella 2.5 have noted results similar to those experienced with Impella 5.0. First, Impella 2.5 was found to improve forward flow in both stable patient and those with cardiogenic shock. For instance, Valgimigli and colleagues noted that cardiac output produced by the LV decreased from 5.99 L/min to 5.01 L/min in a patient undergoing high-risk PCI with Impella 2.5 support. However, total cardiac output as measured by thermodilution increased from 5.95 L/min to 7.36 L/min, showing a 2.41 L/min increase, consistent with the projected flow increase provided by the device at the P9 performance level.22 Similarly, cardiac index was noted to increase 0.49 L/min/m2 within 30 minutes of initiating Impella 2.5 support in 12 cardiogenic shock patients compared to an increase of only 0.11 L/min/m2 in 13 patients placed on IABP support (P=.02).16
Intracoronary pressure has also been shown to increase significantly with the use of Impella 2.5. Aortic and coronary pressures, hyperemic flow velocity, and coronary flow reserve have been shown to increase significantly with increasing Impella support levels in patients undergoing high-risk PCI. In turn, hyperemic microvascular resistance significantly decreases with Impella support, such that increased coronary flow was due both to increased perfusion pressure as well as decreased LV intramyocardial resistance.23 This change in microcirculatory patterns was further demonstrated in patients supported with Impella 2.5 within 6 hours of anterior ST-elevation myocardial infarction (STEMI). Small capillary blood flow and functional capillary density as measured by sidestream dark-field technology significantly improved at 24 hours in patients supported by Impella 2.5, matching quality of flow in healthy matched controls. No improvement was noted in patients without support.24 Additionally, a significant 41% improvement in ejection fraction at 4 months was noted in patients supported by Impella after ST-segment elevation myocardial infarction, significantly more than those supported by IABP.17,24 Although infarct size has been shown in animal studies to be significantly reduced by the use of Impella 5.0,20 this was the first indirect evidence that Impella 2.5 could similarly promote myocardial salvage.
Impella 2.5 has also demonstrated effective support of patients undergoing elective high-risk PCI,12-15,22,25 maintaining stable diastolic blood pressure and MAP even during extended balloon inflations in high-risk lesions. Although initially encouraging, full support for its use in this population was recently called into question. The recent PROTECT-2 study, which randomized high-risk patients to receive IABP vs Impella 2.5, support during high-risk PCI was stopped early after an interim analysis demonstrated the primary endpoint, a composite of 10 major adverse events, did not differ between the two groups (P=.312 in the updated analysis of 447 patients). Although they did not dismiss the findings, the trial investigators surmised that the increased use of rotational atherectomy in patients supported by Impella 2.5 could account for some of the lack of benefit, as a prespecified analysis of patients who did not undergo rotational atherectomy did see a statistically significant improvement in the primary outcome (RR, 0.75; P=.015).26 Given these findings, further studies targeting clinical endpoints are warranted.
Through preliminary experience, it is clear that the function of the Impella 2.5 and its ability to unload the LV is dependent on loading conditions. PV loops from a single patient undergoing high-risk PCI demonstrated the expected leftward shift and reduction in stroke volume (94 to 76 mL) from increasing Impella support.22 However, a follow-up study performed by the same group evaluating PV loops in 8 additional high-risk PCI patients revealed this same trend in only 1 patient while 6 patients demonstrated an unexpected rightward shift.27 However, on examining the raw data, it becomes apparent that baseline end-diastolic volumes were approximately 120 mL lower on average, with concomitantly decreased LVEDP compared to the single patient experience. Additionally, 50% of patients experienced a suction effect against the wall of the LV, relieved by lowering pump speed and infusion of volume. This suggests that proper Impella function is dependent on LV filling parameters, with true LV unloading and maximization of pump flow occurring in those who are not initially euvolemic (Figure 3).
This fundamental concept, which is true for all axial pumps, is based on the fact that flow is indirectly proportional to the pressure difference between the LV and the aorta. For instance, if LVEDP is 40 mm Hg and MAP is 70 mm Hg, axial pumps will provide more flow for the same RPM compared to a scenario where LVEDP is 10 mm Hg and MAP is 110 mm Hg. This knowledge might assist the clinician in optimizing the flow through the pump by manipulating loading conditions and creating a situation where the Impella and the heart are working in concert. For instance, infusion of small volumes of normal saline or blood products when clinically indicated may be helpful when suction events occur with high frequency.
TandemHeart
Technical considerations. The TandemHeart system is a centrifugal pump connecting a femoral venous inlet cannula providing access to the left atrium via a transeptal puncture and a femoral arterial outlet cannula. The device can provide up to 5 L/min of flow and can be used for up to 14 days. After right femoral venous access, a trans-septal puncture is made in a standard fashion and a wire is advanced into the left atrium. After sequentially dilating the atrial septal puncture to 21 Fr, the venous inflow cannula is advanced into the left atrium over a stiff 0.035˝ guidewire. This cannula is constructed with 14 sideholes near its distal tip to facilitate aspiration of blood from the left atrium, a curve to mimic the natural shape of the inferior vena cava-atrium interface, and 3 fluoroscopically visible markings near the tip to assist in placement. Precise placement of the inflow cannula into the mid-left atrium is vital as its proximity to either the superior border of left atrium or the wall of the left superior pulmonary vein could trigger “suction effect,” significant arrhythmia, or subsequent cannula dislodgement secondary to patient movement.
The arterial outflow cannula is then placed using standard techniques with step-wise dilation to support the 15-17 Fr arterial cannula (although two 12 Fr arterial cannulae can be placed in the two femoral arteries). The intermediary centrifugal pump is connected to each cannula via 3/8˝ polyvinyl chloride tubing. The arterial cannula is connected first; once connected, the arterial outflow tubing is unclamped to allow back-bleeding sequentially through the pump and the inflow tubing (which is not yet connected to the trans-septal cannula). Ensuring a secure wet-to-wet connection at this stage is essential in order to avoid air embolism and is aided by 2 barbed connectors on the proximal end of the trans-septal cannula. Once primed, the inflow tubing is connected to the trans-septal cannula via a wet-to-wet, air-tight connection and secured at the first barb (which allows for the tubing to be disconnected if further de-airing is needed), and once all air is removed, the tubing is permanently secured across the second barb. Because connections at this point cannot physically be undone without incision of the cannula tubing across the barb, the authors recommend training from either a perfusionist or cardiac surgeon prior to patient use. The clamp on the inflow tubing is removed and the pump speed is immediately increased to approximately 5000 rpm dependent on patient needs. The pump can deliver up to 5 L/min of blood flow at a motor speed of 7500 rpm.
The pump design features a hydrodynamic fluid bearing fed by 10 cc/hour of dextrose-free heparinized saline that lubricates and supports a spinning rotor, translating into 900 units/hour of heparin delivery to the patient. While the device remains in place, a target ACT of ≥200 seconds, correlating to an aPTT between 65-80 seconds (2.5-3x normal), is maintained. The bedside system controller displays pump motor speed and flow rate via either a flow sensor or derived number based on motor speed. In the event of a suction event against the left atrium, either because of malpositioning of the trans-septal cannula or decreased left atrial volumes, the system will chatter as the cannula comes on and off the atrial wall with systole and diastole, and the console may show a decrease in flow, but the pump speed will not change. This requires a reduction in pump speed to allow for correction of the underlying cause. However, flow rates ≤1.0 L/min should not be maintained for more than 30 minutes, and additional heparin should be considered to avoid the risk of thrombosis. If the decision is made to stop the pump entirely, the outflow arterial tubing should be clamped and additional heparin should be considered.
Outcomes data. Similar to the Impella 2.5, the TandemHeart is capable of providing significant cardiac support and LV unloading. Several studies have demonstrated this effect in cardiogenic shock patients.28-30 For instance, one recent study performed in 117 patients with severe refractory cardiogenic shock despite the use of IABP and/or high-dose vasoactive agents demonstrated a dramatic and statistically significant increase in cardiac index (0.52 L/min/m2 to 3.0 L/min/m2), systolic blood pressure (75 mm Hg to 100 mm Hg), MAP (45 mm Hg to 81 mm Hg), mixed venous oxygen saturation (49% to 69%), and PCWP (31.5 mm Hg to 17.3 mm Hg). Clinically, this resulted in a significant increase in urine output (70 mL/day to 1200 mL/day), and a reduction in lactate production (24 mg/dL to 11 mg/dL). The total mortality at 30 days was 40.2% and 45.3% at 6 months, compared to a mortality of 52-76% previously noted in this population without the use of the TandemHeart.7 However, this mortality data should be interpreted with caution as the study was not meant to compare this endpoint in a randomized fashion. Further, a recent meta-analysis comparing percutaneous LVADs (TandemHeart and Impella 2.5) to IABP did not demonstrate a mortality benefit over IABP at 30 days.31
Hemodynamic support was also seen in high-risk PCI patients. One study examining 5 consecutive patients undergoing PCI of the left main coronary artery or last remaining conduit showed additional support of an average of 3.0 L/min by the TandemHeart, yielding 100% angiographic success.32 Many of the patients, however, had near complete loss of contractility during balloon inflation, visualized angiographically as a lack of aortic valve opening. The TandemHeart was capable of providing support of hemodynamics during this time, which points to a potential unique feature of the TandemHeart — that much like centrifugal surgical VADs, native LV contractility may not be necessary for the TandemHeart to support cardiac function.
The complication profile of the TandemHeart includes complications of the trans-septal puncture (puncture of the aortic root, coronary sinus, or posterior free wall of the right atrium), thromboembolism, bleeding, and limb ischemia. The latter is largely related to the size of the inlet and outlet cannulae. Various studies have shown between 5% and 45% of patients supported by TandemHeart will develop bleeding events including access-site bleeding and groin hematomas, some severe and requiring transfusions in as many as 60% of study participants.28-30,32 These events can be partially circumvented with the use of pre-close procedures. Although recent studies have cited a rate of limb ischemia of 3%,7 significant peripheral vascular disease is a relative contraindication to the insertion of TandemHeart given the size of the arterial cannula. However, the device can be inserted safely if the arterial disease is intervened upon prior to device implantation or via antegrade femoral cannulation, both methods utilized by high-volume TandemHeart users. One additional consideration is the time for insertion. IABP insertion was noted to take 11.5 minutes on average, compared to 25 to 65 minutes for TandemHeart in patients with cardiogenic shock.30 Although partially related to level of experience with the mechanical set up, the trans-septal puncture adds a step requiring time and experience that is not necessary for either IABP or Impella 2.5. This, however, may be improved with continued device experience and has not necessarily resulted in a detriment to clinical outcomes to date.
Future Directions
Right ventricular assist device. Although right ventricular (RV) failure from acute myocardial infarction only accounts for 2.8% cases of cardiogenic shock, the mortality approaches 60%.33 Decreased RV function has been shown to be an independent predictor of total and cardiovascular mortality. Zornoff et al demonstrated that for each 5% decrease in fractional area change in the RV, there was a 16% increased odds of cardiovascular mortality.34
There has been demonstrated utility of Impella 2.5 and TandemHeart for LV failure, but support of the acutely failing RV is only minimally reported. For instance, case reports demonstrate the use of TandemHeart to provide RV support for pulmonary hypertension and for cardiogenic shock after right coronary artery infarct.35-39 The most recent described a patient who presented with acute inferoposterior MI with multivessel coronary disease and thrombotic occlusion of the left circumflex artery resulting in cardiogenic shock requiring multivessel PCI.39 Despite successful intervention, the patient’s hemodynamic status did not substantially improve secondary to RV failure. The TandemHeart was placed using two 75 cm, 21 Fr cannulae, one serving as the outflow from the right atrium and the other the inflow to the pulmonary artery. Within 24 hours of RVAD placement, the patient’s hemodynamics improved with reduction in heart rate from 87 bpm to 77 bpm, reduction in central venous pressure from 14 mm Hg to 7 mm Hg, and increase in blood pressure from 87/57 mm Hg to 117/68 mm Hg. Vasoactive agents were weaned two days after RVAD placement and the patient was successfully extubated. The patient was discharged home on hospital day 7. Serial echocardiograms to evaluate his cardiac function 1 year after this event noted an ejection fraction of 61% with normal RV performance.
The Impella design has also been recently modified for specific use in the failing RV. The Impella Right Peripheral (RP) device is a percutaneous catheter-mounted axial flow pump, much like its left-sided precursor. It is placed via the right femoral vein across the tricuspid valve and into the pulmonary artery, providing greater than 4 L of flow from the lower right atrium to the pulmonary artery. Its specific uses target right heart dysfunction specifically, whether acute or chronic in etiology. September 2010 marked the first in-man experience in Canada where the device was placed for acute right heart dysfunction after heart transplantation and supported the patient successfully for 6 days. The device has limited availability currently, under “special access” regulations in Canada, and is not yet available in the United States.
Conclusion
Impella 2.5 and TandemHeart devices have provided percutaneous operators with options for hemodynamic support previously only available in the operating room via surgical VADs. In the correct patient population, these devices have consistently shown improvements in forward flow and LV unloading with relative ease of use. Although robust improvements in clinical outcomes remain in question, their availability offers the clinician advanced percutaneous tools for hemodynamic support.
References
- DeBakey ME. Left ventricular bypass pump for cardiac assistance. Clinical experience. Am J Cardiol. 1971;27(1):3-11.
- Moulopoulos SD, Topaz S, Kolff WJ. Diastolic balloon pumping (with carbon dioxide) in the aorta — a mechanical assistance to the failing circulation. Am Heart J. 1962;63:669-675.
- Kantrowitz A, Tjonneland S, Freed PS, Phillips SJ, Butner AN, Sherman JL Jr. Initial clinical experience with intraaortic balloon pumping in cardiogenic shock. JAMA. 1968;203(2):113-118.
- Bregman D. A new percutaneous intra-aortic balloon. Trans Am Soc Artif Intern Organs. 1980;26:8-11.
- Weber KT, Janicki JS. Intraaortic balloon counterpulsation. A review of physiological principles, clinical results, and device safety. Ann Thorac Surg. 1974;17(6):602-636.
- Scheidt S, Wilner G, Mueller H, et al. Intra-aortic balloon counterpulsation in cardiogenic shock. Report of a co-operative clinical trial. N Engl J Med. 1973;288(19):979-984.
- Kar B, Gregoric ID, Basra SS, Idelchik GM, Loyalka P. The percutaneous ventricular assist device in severe refractory cardiogenic shock. J Am Coll Cardiol. 2011;57(6):688-696.
- Perera D, Stables R, Thomas M, et al. Elective intra-aortic balloon counterpulsation during high-risk percutaneous coronary intervention: a randomized controlled trial. JAMA. 2010;304(8):867-874.
- Goldstein D, Zucker M, Pagani F, et al. In: Mechanical Circulatory Support. Philadelphia: Elsevier, Inc., 2006: Chapter 5.
- Antaki J, Poirier V, Pagani F. In: Mechanical Circulatory Support. Philadelphia: Elsevier Inc., 2006: Chapter 3.
- Mehrotra AK, Shah D, Sugeng L, et al. Echocardiography for percutaneous heart pumps. JACC Cardiovasc Imaging. 2009;2(11):1332-1333.
- Burzotta F, Paloscia L, Trani C, et al. Feasibility and long-term safety of elective Impella-assisted high-risk percutaneous coronary intervention: a pilot two-centre study. J Cardiovasc Med (Hagerstown). 2008;9(10):1004-1010.
- Dixon SR, Henriques JP, Mauri L, et al. A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (the PROTECT I trial): initial U.S. experience. JACC Cardiovasc Interv. 2009;2(2):91-96.
- Ferreiro JL, Gomez-Hospital JA, Cequier AR, et al. Use of Impella Recover LP 2.5 in elective high risk percutaneous coronary intervention. Int J Cardiol. 2010;145(2):235-237.
- Henriques JP, Remmelink M, Baan J Jr, et al. Safety and feasibility of elective high-risk percutaneous coronary intervention procedures with left ventricular support of the Impella Recover LP 2.5. Am J Cardiol. 2006;97(7):990-992.
- Seyfarth M, Sibbing D, Bauer I, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52(19):1584-1588.
- Sjauw KD, Remmelink M, Baan J Jr, et al. Left ventricular unloading in acute ST-segment elevation myocardial infarction patients is safe and feasible and provides acute and sustained left ventricular recovery. J Am Coll Cardiol. 2008;51(10):1044-1046.
- Meyns B, Dens J, Sergeant P, et al. Initial experiences with the Impella device in patients with cardiogenic shock — Impella support for cardiogenic shock. Thorac Cardiovasc Surg. 2003;51(6):312-317.
- Reesink KD, Dekker AL, Van Ommen V, et al. Miniature intracardiac assist device provides more effective cardiac unloading and circulatory support during severe left heart failure than intraaortic balloon pumping. Chest. 2004;126(3):896-902.
- Meyns B, Stolinski J, Leunens V, et al. Left ventricular support by catheter-mounted axial flow pump reduces infarct size. J Am Coll Cardiol. 2003;41(7):1087-1095.
- Sauren LD, Accord RE, Hamzeh K, et al. Combined Impella and intra-aortic balloon pump support to improve both ventricular unloading and coronary blood flow for myocardial recovery: an experimental study. Artif Organs. 2007;31(11):839-842.
- Valgimigli M, Steendijk P, Sianos G, et al. Left ventricular unloading and concomitant total cardiac output increase by the use of percutaneous Impella Recover LP 2.5 assist device during high-risk coronary intervention. Catheter Cardiovasc Interv. 2005;65(2):263-267.
- Remmelink M, Sjauw KD, Henriques JP, et al. Effects of left ventricular unloading by Impella recover LP2.5 on coronary hemodynamics. Catheter Cardiovasc Interv. 2007;70(4):532-537.
- Lam K, Sjauw KD, Henriques JP, et al. Improved microcirculation in patients with an acute ST-elevation myocardial infarction treated with the Impella LP2.5 percutaneous left ventricular assist device. Clin Res Cardiol. 2009;98(5):311-318.
- Jolly N. Role of Impella 2.5 heart pump in stabilizing diastolic aortic pressure to avert acute hemodynamic collapse during coronary interventions. J Invasive Cardiol. 2009;21(7):E134-E136.
- Neale T. ACC: Heart Pump Still Promising After Missing Endpoint [American College of Cardiology: CardioSource website]. April 5, 2011. Available at: https://www.medpagetoday.com/MeetingCoverage/ACC/25719. Accessed April 30, 2011.
- Valgimigli M, Steendijk P, Serruys PW, et al. Use of Impella Recover(R) LP 2.5 left ventricular assist device during high-risk percutaneous coronary interventions; clinical, haemodynamic and biochemical findings. EuroIntervention. 2006;2(1):91-100.
- Burkhoff D, Cohen H, Brunckhorst C, et al. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J. 2006;152(3):469.e1-8.
- Burkhoff D, O’Neill W, Brunckhorst C, et al. Feasibility study of the use of the TandemHeart percutaneous ventricular assist device for treatment of cardiogenic shock. Catheter Cardiovasc Interv. 2006;68(2):211-217.
- Thiele H, Sick P, Boudriot E, et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26(13):1276-1283.
- Cheng JM, den Uil CA, Hoeks SE, et al. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Eur Heart J. 2009;30(17):2102-2108.
- Kar B, Forrester M, Gemmato C, et al. Use of the TandemHeart Percutaneous Ventricular Assist Device to Support Patients Undergoing High-Risk Percutaneous Coronary Intervention. J Invasive Cardiol. 2006;18(4):A6.
- Hochman JS, Buller CE, Sleeper LA, et al. Cardiogenic shock complicating acute myocardial infarction--etiologies, management and outcome: a report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK? J Am Coll Cardiol. 2000;36(3 Suppl A):1063-1070.
- Zornoff LA, Skali H, Pfeffer MA, et al. Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J Am Coll Cardiol. 2002;39(9):1450-1455.
- Atiemo AD, Conte JV, Heldman AW. Resuscitation and recovery from acute right ventricular failure using a percutaneous right ventricular assist device. Catheter Cardiovasc Interv. 2006;68(1):78-82.
- Kiernan MS, Krishnamurthy B, Kapur NK. Percutaneous right ventricular assist via the internal jugular vein in cardiogenic shock complicating an acute inferior myocardial infarction. J Invasive Cardiol. 2010;22(2):E23-E26.
- Prutkin JM, Strote JA, Stout KK. Percutaneous right ventricular assist device as support for cardiogenic shock due to right ventricular infarction. J Invasive Cardiol. 2008;20(7):E215-E216.
- Rajdev S, Benza R, Misra V. Use of Tandem Heart as a temporary hemodynamic support option for severe pulmonary artery hypertension complicated by cardiogenic shock. J Invasive Cardiol. 2007;19(8):E226-E229.
- Weiss S, Jolly N, Shah A. Multivessel intervention and placement of a percutaneous right ventricular assist device in a patient with acute myocardial infarction complicated by cardiac arrest. J Invasive Cardiol. 2011;23(6):248-251.
_______________________________________
From the Section of Cardiology, University of Chicago Medical Center, Chicago, Illinois.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Jolly has served on the scientific advisory board of Abiomed. Dr. Weiss reported no disclosures. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted August 19, 2011, provisional acceptance given September 12, 2011, final version accepted September 20, 2011.
Address for correspondence: Dr Neeraj Jolly, Rush University Medical Center, Section of Cardiology – 1017 Jelke, 1653 W. Congress Parkway, Chicago IL 60612-3833. Email: neeraj_jolly@rush.edu
















