Percutaneous Pulmonary Valve Implantation in Left Pulmonary Artery Branch in a Patient with a Functional Single Lung
ABSTRACT: Percutaneous pulmonary valve technology has had a great impact on patients with congenital and acquired heart disease. In some patients, implantation of a percutaneous pulmonary valve may not be possible due to the morphology of the existing right ventricular outflow tract. In this report, we describe implantation of a Melody transcatheter pulmonary valve in the left pulmonary artery in a patient with acquired right pulmonary artery occlusion and a large right ventricular outflow tract.
J INVASIVE CARDIOL 2012;24(9):E202-E204
Key words: transcatheter, percutaneous, pulmonary valve, pulmonary artery
___________________________________________________
Since the first report of a percutaneously implanted pulmonary valve in a human,1 there have been approximately 2,500 reported implants of the Melody valve (Medtronic) worldwide through July 2011 (personal communication, Medtronic). The indications for implantation of this valve in the catheterization laboratory are specific for right ventricle to pulmonary artery conduits with significant stenosis and/or regurgitation. In this article, we report transcatheter placement of the Melody valve in the proximal left pulmonary artery of a patient with acquired right pulmonary artery occlusion, in whom the existing pulmonary homograft was too large to allow for placement of a percutaneous pulmonary valve.
Case Report. A 58-year-old male was diagnosed with a rare form of pulmonary artery sarcoma that involved his right pulmonary artery (RPA) and right ventricular outflow tract. He underwent resection of the pulmonary artery sarcoma and reconstruction of his RPA with an 18 mm aortic non-valved homograft. His right ventricular outflow tract was augmented with a 31 mm pulmonary homograft. His subsequent management included chemotherapy and radiation therapy. Due to progressive fatigue and respiratory symptoms, he was admitted to the hospital 2 years later. A work-up, including a CT scan of the chest, revealed total occlusion of his proximal RPA. An attempt to recanalize this vessel in the catheterization laboratory was unsuccessful at that time. The patient went on to develop pulmonary hypertension in the left lung and was treated with oral pulmonary vasodilators. With medical management he did well for a few years; however he recently became progressively more fatigued with limiting dyspnea on exertion. In addition, he had severe pulmonary regurgitation. A cardiac MRI, 7 years after his initial surgery, showed a mildly dilated right ventricle, but severe pulmonary regurgitation with a regurgitant fraction of 50% and a diminished right ventricular ejection fraction of 43%. His pulmonary artery homograft measured 34 mm at its narrowest point and his distal RPA beyond the occlusion was not visualized. The left pulmonary artery (LPA) measured approximately 18 mm-20 mm and the distance from the main pulmonary artery (MPA)/LPA junction to the origin of the left upper lobe pulmonary artery branch was 26 mm. Given his constellation of symptoms with pulmonary hypertension in the setting of severe pulmonary regurgitation, we felt that placement of a competent pulmonary valve would be beneficial. He was considered a high-risk surgical candidate due to the combination of extensive scar tissue from radiation and prior surgery. After obtaining approval from our Institutional Review Board, he was taken to the catheterization laboratory for placement of a Melody valve (Medtronic) in the proximal LPA, if his RPA was not amenable to percutaneous recanalization.
 The procedure was performed under general anesthesia and heparin was administered to achieve an activated clotting time of >250 seconds. Femoral venous and arterial access was obtained. His estimated cardiac index was 1.9 L/min/m2. His pulmonary artery pressure was 60/26, 35 mm Hg with a systemic arterial pressure of 127/68, 92 mm Hg, and an indexed pulmonary vascular resistance of 8 Wood units. An aortogram showed some pulmonary venous return on the levophase to the right-sided pulmonary veins, but no definite distal RPA. We obtained transseptal access to the left atrium and performed wedge angiograms in the right upper and right lower pulmonary veins. These angiograms demonstrated a diminutive (<1 mm diameter) distal RPA that made recanalization of the RPA not feasible.
The procedure was performed under general anesthesia and heparin was administered to achieve an activated clotting time of >250 seconds. Femoral venous and arterial access was obtained. His estimated cardiac index was 1.9 L/min/m2. His pulmonary artery pressure was 60/26, 35 mm Hg with a systemic arterial pressure of 127/68, 92 mm Hg, and an indexed pulmonary vascular resistance of 8 Wood units. An aortogram showed some pulmonary venous return on the levophase to the right-sided pulmonary veins, but no definite distal RPA. We obtained transseptal access to the left atrium and performed wedge angiograms in the right upper and right lower pulmonary veins. These angiograms demonstrated a diminutive (<1 mm diameter) distal RPA that made recanalization of the RPA not feasible.
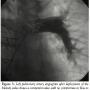
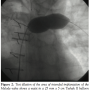 We then proceeded to evaluate the LPA for implantation of the Melody valve (Medtronic). An angiogram in the MPA/proximal LPA region showed occlusion of the proximal RPA and free pulmonary regurgitation with the proximal LPA measuring 18 mm, increasing to 20 mm prior to the origin of the left upper lobe pulmonary artery branch (Figure 1). The distance from the MPA/LPA junction to the origin of the left upper lobe pulmonary artery was confirmed at 26 mm. In order to test the compliance of the lesion and to better assess the size of the narrowest portion of the proximal LPA, a 25 mm x 5 cm Tyshak II balloon (B. Braun) was advanced to the proximal LPA and inflated at low pressure to 2 atm. The waist on the balloon measured 20 mm and the balloon remained in stable position during this inflation (Figure 2). The narrowest portion was at the MPA/proximal LPA, at the previous surgical suture line with the pulmonary artery homograft. We decided to deploy the Melody valve in this area using a 22 mm Ensemble Transcatheter Delivery System (Medtronic). A curved 0.035” Lunderquist Extra Stiff Wire Guide (Cook Medical) was placed in the distal LPA and the system was advanced with relative ease to the LPA where it was positioned. Multiple angiograms through the side port were performed to accurately position the valve and place it proximal to the origin of the left upper lobe pulmonary artery. The inner and outer balloons were inflated sequentially and then deflated. The outer balloon was then inflated to slightly higher pressure. There was a residual waist seen in the mid portion of the stent measuring about 20 mm at the origin of the LPA from the MPA. Pullback pressures demonstrated a 4 mm Hg systolic gradient from the distal LPA to the MPA across the valve. Repeat angiography showed excellent valve function with no compromise in flow to the left upper lobe branch pulmonary artery and no regurgitation (Figure 3). A transthoracic echocardiogram the next day confirmed excellent valve function with trivial regurgitation through the valved stent.
We then proceeded to evaluate the LPA for implantation of the Melody valve (Medtronic). An angiogram in the MPA/proximal LPA region showed occlusion of the proximal RPA and free pulmonary regurgitation with the proximal LPA measuring 18 mm, increasing to 20 mm prior to the origin of the left upper lobe pulmonary artery branch (Figure 1). The distance from the MPA/LPA junction to the origin of the left upper lobe pulmonary artery was confirmed at 26 mm. In order to test the compliance of the lesion and to better assess the size of the narrowest portion of the proximal LPA, a 25 mm x 5 cm Tyshak II balloon (B. Braun) was advanced to the proximal LPA and inflated at low pressure to 2 atm. The waist on the balloon measured 20 mm and the balloon remained in stable position during this inflation (Figure 2). The narrowest portion was at the MPA/proximal LPA, at the previous surgical suture line with the pulmonary artery homograft. We decided to deploy the Melody valve in this area using a 22 mm Ensemble Transcatheter Delivery System (Medtronic). A curved 0.035” Lunderquist Extra Stiff Wire Guide (Cook Medical) was placed in the distal LPA and the system was advanced with relative ease to the LPA where it was positioned. Multiple angiograms through the side port were performed to accurately position the valve and place it proximal to the origin of the left upper lobe pulmonary artery. The inner and outer balloons were inflated sequentially and then deflated. The outer balloon was then inflated to slightly higher pressure. There was a residual waist seen in the mid portion of the stent measuring about 20 mm at the origin of the LPA from the MPA. Pullback pressures demonstrated a 4 mm Hg systolic gradient from the distal LPA to the MPA across the valve. Repeat angiography showed excellent valve function with no compromise in flow to the left upper lobe branch pulmonary artery and no regurgitation (Figure 3). A transthoracic echocardiogram the next day confirmed excellent valve function with trivial regurgitation through the valved stent.
 The patient developed new onset atrial fibrillation one week after the procedure (presumably from catheter manipulation in the left atrium) that was treated with beta-blockers. He spontaneously reverted to normal sinus rhythm and has otherwise done well in the 9-month period following his intervention with gradually improving dyspnea and fatigue. His right ventricle (RV) size has decreased at latest follow-up with improvement in function by echocardiography.
The patient developed new onset atrial fibrillation one week after the procedure (presumably from catheter manipulation in the left atrium) that was treated with beta-blockers. He spontaneously reverted to normal sinus rhythm and has otherwise done well in the 9-month period following his intervention with gradually improving dyspnea and fatigue. His right ventricle (RV) size has decreased at latest follow-up with improvement in function by echocardiography.
Discussion. The Melody transcatheter pulmonary valve (Medtronic) has a CE Mark in Europe and is approved by the FDA in the U.S. with humanitarian device exemption status. The valve has specific indications for implantation in patients with significant pulmonary regurgitation and/or stenosis in whom the originally implanted RV-PA conduit was at least 16 mm in diameter and the waist on a sizing balloon in the catheterization laboratory is >14 mm <20 mm. When used under these circumstances, the experience in Europe and the U.S. with the Melody valve has shown that implantation is technically feasible, with good short- and intermediate-term results with regards to valve function and its physiologic impact.2-9
Pulmonary valve replacement has been shown to be beneficial in the setting of pulmonary hypertension.10 In our patient, his existing conduit was too large to allow for percutaneous placement of the Melody valve. An extensive and careful evaluation was undertaken and due to the fact that he was considered a high-risk surgical candidate, we elected to implant the valve in his LPA, which physiologically is his right ventricular outflow tract in the setting of a one-lung circulation. The Melody valve has been implanted in the branch pulmonary arteries in an animal model11 and has been shown to decrease the pulmonary regurgitant fraction and improve ventricular function. However, as pointed out by Robb et al,11 the impact of future regurgitation on the native right ventricular outflow tract and its contribution to overall right ventricular size and function remains unknown.
Pre-catheterization assessment by MRI (or CT when applicable) prior to percutaneous implantation of pulmonary valves is helpful for pre-procedure planning. The predicted length from the MPA to the left upper lobe origin was deemed adequate by MRI and reconfirmed by angiography. This is an important consideration, as the Melody valve is a covered stent, and we did not want to cover any of the left pulmonary artery branches. We deliberately deployed the very proximal portion of the Melody valve in the MPA, not only to center the stent, but also to avoid covering the origin of the left upper lobe pulmonary artery. Another important consideration is the use of pre-dilation. Pre-dilation not only allowed us to evaluate the compliance of the vessel at the implantation site, but also allowed us to assess stability of the balloon, and thus gauge stability of the Melody valve during deployment. Stability of any stent may be an issue in the absence of sufficient stenosis in the face of regurgitation. In our case, the fact that there was a mild narrowing at the MPA/LPA junction from previous surgery was advantageous, as it allowed for an “anchoring zone” for our stent. Since there was adequate stability during this maneuver, we did not feel the need to rapidly pace the RV for stability of the valve. However, this should be considered in circumstances like ours12 if there is any concern for excessive movement during test dilation or if a focal narrowing is not present.
In humans, the use of the Melody valve has now been extended to implantation in right ventricular outflow tracts that have been previously patched13 and in the tricuspid valve position.14 Research is ongoing on implantation of percutaneous pulmonary valves in native outflow tracts as well.15 It is clear that a single valved stent design is not applicable to all patients with dysfunctional right ventricular outflow tracts. The development of valve technologies that are applicable to large, native, and outflow tracts of various anatomic morphologies holds great promise for many of our patients. While the routine use of the Melody valve in a branch pulmonary artery is not to be proposed, as seen in this report, this modality is technically feasible and may be considered an option in high-risk patients under special circumstances.
References
- Bonhoeffer P, Boudjemline Y, Saliba Z, et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;21(9239):1403-1405.
- Bonhoeffer P, Boudjemline Y, Qureshi SA, et al. Percutaneous insertion of the pulmonary valve. J Am Coll Cardiol. 2002;39(10):1664-1669.
- Khambadkone S, Coats L, Taylor A, et al. Percutaneous pulmonary valve implantation in humans: results in 59 consecutive patients. Circulation. 2005;112(8):1189-1197.
- Zahn EM, Hellenbrand WE, Lock JE, McElhinney DB. Implantation of the melody transcatheter pulmonary valve in patients with a dysfunctional right ventricular outflow tract conduit early results from the U.S. Clinical trial. J Am Coll Cardiol. 2009;54(18):1722-1729.
- McElhinney DB, Hellenbrand WE, Zahn EM, et al. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation. 2010;122(5):507-516.
- Lurz P, Coats L, Khambadkone S, et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117(15):1964-1972.
- Lurz P, Giardini A, Taylor AM, et al. Effect of altering pathologic right ventricular loading conditions by percutaneous pulmonary valve implantation on exercise capacity. Am J Cardiol. 2010;105(5):721-726.
- Lurz P, Nordmeyer J, Giardini A, et al. Early versus late functional outcome after successful percutaneous pulmonary valve implantation: are the acute effects of altered right ventricular loading all we can expect? J Am Coll Cardiol. 2011;57(6):724-731.
- Lurz P, Bonhoeffer P. Percutaneous implantation of pulmonary valves for treatment of right ventricular outflow tract dysfunction. Cardiol Young. 2008;18(3):260-267.
- Lurz P, Nordmeyer J, Coats L, Taylor AM, Bonhoeffer P, Schulze-Neick I. Immediate clinical and haemodynamic benefits of restoration of pulmonary valvar competence in patients with pulmonary hypertension. Heart. 2009;95(8):646-650.
- Robb JD, Harris MA, Minakawa M, et al. Melody valve implantation into the branch pulmonary arteries for treatment of pulmonary insufficiency in an ovine model of right ventricular outflow tract dysfunction following tetralogy of Fallot repair. Circ Cardiovasc Interv. 2011;4(1):80-87.
- Mehta C, Desai T, Shebani S, Stickley J, De Giovanni J. Rapid ventricular pacing for catheter interventions in congenital aortic stenosis and coarctation: effectiveness, safety, and rate titration for optimal results. J Interv Cardiol. 2010;23(1):7-13.
- Momenah TS, El Oakley R, Al Najashi K, Khoshhal S, Al Qethamy H, Bonhoeffer P. Extended application of percutaneous pulmonary valve implantation. J Am Coll Cardiol. 2009;53(20):1859-1863.
- Roberts PA, Boudjemline Y, Cheatham JP, et al. Percutaneous tricuspid valve replacement in congenital and acquired heart disease. J Am Coll Cardiol. 2011;58(2):117-122.
- Boudjemline Y, Agnoletti G, Bonnet D, Sidi D, Bonhoeffer P. Percutaneous pulmonary valve replacement in a large right ventricular outflow tract: an experimental study. J Am Coll Cardiol. 2004;43(6):1082-1087.
___________________________________________________
From the Center for Pediatric and Adult Congenital Heart Disease, The Cleveland Clinic, Cleveland, Ohio.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. All authors report travel reimbursement for a Medtronic training course. Dr Krasuski is a consultant (Gilead and Actelion), serves on Actelion's Speaker's Bureau, and has received honoraria from Actelion.
Manuscript submitted April 2, 2012 and accepted May 1, 2012.
Address for correspondence: Athar M. Qureshi, MD, Desk M-41, Center for Pediatric and Congenital Heart Disease, The Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195, USA. Email: quresha@ccf.org
















