Percutaneous Coronary Vein Angioplasty for Severe Spasm of the Posterolateral Vein During Bi-Ventricular Pacing
ABSTRACT: Coronary venous spasm is an under-recognized and often reversible technical challenge that may be encountered while placing bi-ventricular pacing devices. We describe a case of severe vasopsam in the posterolateral left ventricular vein that was managed by balloon angioplasty, followed by successful placement of the left ventricular lead.
J INVASIVE CARDIOL 2011;23:E170–E172
______________________________________
Promising results with cardiac resynchronization (CRT) have been reported in selected patients with impaired ventricular function and left bundle branch block (LBBB).1,2 Technical challenges like variations in coronary venous anatomy, lack of adequate target veins, venous dissection, and rarely, venous spasm, may preclude successful placement of the left ventricular (LV) lead. Though recent reports have described the use of balloon angioplasty during CRT procedures, almost all these have been for dilatation of coronary venous stenosis, and rarely for stabilizing unstable leads by stenting within the target vein. Spasm of the coronary sinus (CS) or its tributaries is an under-recognized and often reversible challenge that one may encounter. We describe a case where severe spasm of the posterolateral vein (PLV) occurred during placement of the LV lead, which was successfully managed by balloon angioplasty.
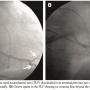 Case Report. A 48-year-old man with idiopathic dilated cardiomyopathy (ejection fraction: 25%), symptomatic congestive heart failure, and LBBB (QRS of 165 ms) was taken up for CRT. Triple subclavian punctures were performed and the CS was cannulated using a deflectable decapolar catheter (Cordis Corporation, Miami Lakes, Florida). Contrast angiography showed an adequate caliber PLV that divided terminally into two subdivisions, one oriented toward the diaphragm and the other coursing cranially (Figure 1A).
Case Report. A 48-year-old man with idiopathic dilated cardiomyopathy (ejection fraction: 25%), symptomatic congestive heart failure, and LBBB (QRS of 165 ms) was taken up for CRT. Triple subclavian punctures were performed and the CS was cannulated using a deflectable decapolar catheter (Cordis Corporation, Miami Lakes, Florida). Contrast angiography showed an adequate caliber PLV that divided terminally into two subdivisions, one oriented toward the diaphragm and the other coursing cranially (Figure 1A).
 An over-the-wire LV lead (Corox OTW 85-BP; Biotronik, Portland, Oregon) was manipulated over a 0.014˝ Cougar angioplasty wire (Medtronic Inc., Minneapolis, Minnesota); however, orientation of the lead even slightly toward the inferior division of the PLV, expectedly resulted in diaphragmatic stimulation at outputs > 3.5 V, despite good parameters (R wave of 20 mV; threshold, 0.8 V). Attempts to place the lead in the cranially oriented tributary resulted in satisfactory parameters (R wave of 18 mV; threshold, 0.7 V) without any diaphragmatic stimulation. Since the distal caliber of this tributary was inadequate to accommodate the entire length of the lead tip, we had no choice but to only partially wedge the lead tip into this tributary. Following this, the right atrial and right ventricular leads were placed routinely with satisfactory parameters. However, during withdrawal of the long sheath of the LV lead, the lead got dislodged from its position.
An over-the-wire LV lead (Corox OTW 85-BP; Biotronik, Portland, Oregon) was manipulated over a 0.014˝ Cougar angioplasty wire (Medtronic Inc., Minneapolis, Minnesota); however, orientation of the lead even slightly toward the inferior division of the PLV, expectedly resulted in diaphragmatic stimulation at outputs > 3.5 V, despite good parameters (R wave of 20 mV; threshold, 0.8 V). Attempts to place the lead in the cranially oriented tributary resulted in satisfactory parameters (R wave of 18 mV; threshold, 0.7 V) without any diaphragmatic stimulation. Since the distal caliber of this tributary was inadequate to accommodate the entire length of the lead tip, we had no choice but to only partially wedge the lead tip into this tributary. Following this, the right atrial and right ventricular leads were placed routinely with satisfactory parameters. However, during withdrawal of the long sheath of the LV lead, the lead got dislodged from its position.
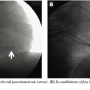
 The CS was recannulated, and while attempting to pass the pacing lead in the PLV, severe resistance was encountered; the lead could not be passed beyond the mid portion of the PLV. Contrast injection revealed severe spasm in the vein with no distal flow beyond the mid PLV (Figure 1B). When repeated injections of nitroglycerin (NTG) failed to restore flow, we decided to perform balloon angioplasty of the vein to restore flow. Low-pressure (4–6 atm) dilatations were performed using a 3.0 x 12 mm Sprinter balloon (Medtronic Inc.) after which flow was restored (Figures 2A and 2B). The cranially oriented tributary of the PLV was also dilated, and this time the lead could be easily placed in this vein, with acceptable parameters, without any diaphragmatic stimulation (Figure 3).
The CS was recannulated, and while attempting to pass the pacing lead in the PLV, severe resistance was encountered; the lead could not be passed beyond the mid portion of the PLV. Contrast injection revealed severe spasm in the vein with no distal flow beyond the mid PLV (Figure 1B). When repeated injections of nitroglycerin (NTG) failed to restore flow, we decided to perform balloon angioplasty of the vein to restore flow. Low-pressure (4–6 atm) dilatations were performed using a 3.0 x 12 mm Sprinter balloon (Medtronic Inc.) after which flow was restored (Figures 2A and 2B). The cranially oriented tributary of the PLV was also dilated, and this time the lead could be easily placed in this vein, with acceptable parameters, without any diaphragmatic stimulation (Figure 3).
Discussion. Challenges during placement of the LV lead may arise due to variations in coronary venous anatomy, ostial valves, CS or branch vein dissections, phrenic nerve stimulation, and rarely coronary venous stenoses.3–5
Though the target vein in our case appeared straightforward, it divided into two terminal tributaries, one angulated cranially and the other caudally toward the diaphragm. Expectedly, attempts at placing the lead in the lower subdivision resulted in diaphragmatic stimulation. Though placement of the lead in the cranially oriented tributary revealed acceptable parameters without any diaphragmatic stimulation, it got dislodged while withdrawing the long sheath. Possibly, since the caliber of this tributary in its distal part was not adequate to accommodate the entire distal tip, and the lead could only be partially wedged into this vein, during sheath withdrawal, the weight of the lead dragged it down into the main PL branch.
Probably, excessive wire or lead manipulation in the PLV precipitated vasospasm, which was unresponsive to repeated injections of NTG. Hence, we elected to perform balloon angioplasty for the resistant vasospasm; following low-pressure dilatations, flow was restored and the lead could be placed successfully.
Recent reports have highlighted the occasional need for performing percutaneous interventional procedures within the coronary venous system to overcome technical challenges during CRT. Almost all such cases have been either for balloon dilation of coronary venous stenosis or for stabilizing unstable leads by stenting within the target vein. Balloon angioplasty using compliant, non-compliant, and even cutting balloons has been reported for coronary venous stenoses.5–7 Though low-pressure dilatations are often sufficient, occasionally high-pressure dilatations may be required.8 Stenting within the target vein has also been reported to stabilize previously unstable leads, without affecting the lead performance.9,10 However, caution should be exercised during such interventions and one should not exceed the rated burst pressure of the balloon during inflation to avoid the risk of coronary vein rupture.11
Spasm of the CS or its tributaries due to excessive catheter/wire manipulation represents an under-recognized and often reversible reason for difficulty in placing LV leads. When the pacing lead does not pass the CS smoothly, it is imperative to perform contrast angiography and find out the cause rather than persist with lead manipulation. Even in our case, when we encountered resistance (when none had been there before), we immediately removed the lead and performed a contrast injection, revealing severe spasm.
In such cases, options include direct injection of NTG12 or cautious performance of balloon angioplasty. To the best of our knowledge, there is only one previous case report of balloon angioplasty for CS vasospasm during performance of CRT; however, in that instance, CS vasopsam was managed using a standard venography balloon.13 We successfully tackled the venospasm using the low-profile usual coronary angioplasty balloon, which in our opinion, may be better suited for performing CS or branch angioplasty whenever required.
Conclusion. Cardiologists need to be aware of unusual technical challenges that they may encounter while implanting a bi-ventricular pacing device. Excessive catheter or guidewire manipulation may lead to vasospasm of the CS or its tributaries. Such cases may be successfully managed by direct injection of NTG or performance of balloon angioplasty using regular angioplasty balloons.
Learning Points:
- Spasm of the CS or its tributaries due to excessive catheter/wire manipulation represents an under-recognized and often reversible reason for inability to place LV leads.
- When the LV pacing lead does not pass across the CS or its tributaries smoothly, one should perform contrast angiography to try and find out the cause rather than persist with lead manipulation.
- Coronary venospasm should first be managed with direct injection of NTG. In resistant cases, low-pressure balloon dilations using angioplasty balloons may be helpful.
References
- Auricchio A, Stellbrink C, Block M, et al. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. Circulation 1999;99:2993–3001.
- Gras D, Mabo P, Tang T, et al. Multisite pacing as a supplemental treatment of congestive heart failure: Preliminary results of the Medtronic Inc. InSync study. Pacing Clin Electrophysiol 1998;21:2249–2255.
- Gilard M, Mansourati J, Etienne Y, et al. Angiographic anatomy of the coronary sinus and its tributaries. Pacing Clin Electrophysiol 1998;21:2280–2284.
- Hansky B, Vogt J, Gueldner H, et al. Left heart pacing — Experience with several types of coronary vein leads. J Interv Card Electrophysiol 2002;6:71–75.
- Hansky B, Lamp B, Minami K, et al. Coronary vein balloon angioplasty for left ventricular pacemaker lead implantation. J Am Coll Cardiol 2002;40:2144–2149.
- Lopez JA, Hernandez E. Transvenous implantation of a coronary sinus lead for left ventricular pacing after cutting balloon angioplasty. Pacing Clin Electrophysiol 2007;30:568–570.
- Chauhan K, Sayad D, Bowerman R, Barold SS. Coronary vein angioplasty with noncompliant balloon for resistant coronary vein stenosis during left ventricular lead implantation. PACE 2008;31:251–252.
- Dauber K, Kaye G. High-pressure balloon angioplasty of coronary sinus vein. Europace 2008;10:1118–1120.
- Szilagyi S, Merkely B, Roka A, et al. Stabilization of the coronary sinus electrode position with coronary stent implantation to prevent and treat dislocation. J Cardiovasc Electrophysiol 2007;18:303–307.
- Kowalski O, Lenarczyk R, Prokopczuk J, et al. Effect of percutaneous interventions within the coronary sinus on the success rate of the implantations of resynchronization pacemakers. Pacing Clin Electrophysiol 2006;29:1075–1080.
- Russo V, Crescenzo I, Ammendola E, et al. Coronary sinus spasm during left ventricular lead implantation for bi-ventricular pacing. Europace 2007;9:528–530.
- Woollett I, Pinney S, Magnano AR. Balloon dilatation of coronary sinus spasm during placement of a bi-ventricular pacing lead. Circulation 2005;111:E304–E305.
- Worley SJ, Gohn DC, Pulliam RW. Coronary vein rupture during venoplasty for LV lead placement. Pacing Clin Electrophysiol 2008;31:904–907.
______________________________________
From the Department of Cardiology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, India.
The authors report no conflicts of interest regarding the content herein.
Manuscript submitted November 2, 2010, provisional acceptance given November 8, 2010, final version accepted November 17, 2010.
Address for correspondence: Dr. Aditya Kapoor, Assistant Professor, Cardiology, Sanjay Gandhi PGIMS, Lucknow 226014, India. Email: akapoor65@gmail.com


















