Patent Hemostasis Impact in Clinical Routine: Large Monocentric Echo-Doppler Study of Radial Artery Patency After Coronary Catheterization
Abstract
Background. Radial access is currently the first line of access in interventional cardiology. Nevertheless, this technique carries a 1%-10% risk of radial artery occlusion (RAO) based on series. Methods. We conducted a large-scale echo-Doppler evaluation of radial patency including prospectively all patients who underwent coronary angiography and/or angioplasty with radial catheterization at our center in 2018. Results. A total of 1106 patients were enrolled in the cohort. Average patient age was 70 years, 24.5% were females and 75.5% were males, and 28.3% were diabetics. Five Fr and 6 Fr devices were used in 527 procedures and 565 procedures, respectively. Our study highlighted a very low RAO rate (0.99%). These results are mainly due to the high attention given to patent hemostasis, which was achieved in 1091 patients (98.6%). According to the literature, female gender is a multivariate predictive factor of RAO (P<.01). Furthermore, we confirm the protective nature of heparin (P=.04) with an average heparin dose of 69.73 IU/kg. Conclusions. Our study focused on a large population with 1106 patients who underwent radial catheterization shows that a very low rate of RAO (0.99%) can be achieved. These results are correlated with a high attention to patent hemostasis and a close collaboration between the medical and paramedical staff.
J INVASIVE CARDIOL 2021;33(2):E77-E82. Epub 2021 Jan 7. doi:10.25270/jic/19.00338
Key words: patent hemostasis, radial access, radial artery occlusion, RAO
Radial access has reduced the incidence of hemorrhagic1 vascular complications2 and postcoronary angiography and coronary angioplasty mortality rates.3 It is now used for first-line access in interventional cardiology. Complications with this access are very rare, and radial artery occlusion (RAO) is considered the “Achilles heel” of the radial approach.4 The incidence of this complication varies from 1%-10%, depending on the series.5-9 Some studies have highlighted an occlusion rate of up to 33%.10 These variations in terms of incidence probably depend on diagnostic methods.6,9 Although most RAOs remain asymptomatic thanks to the extensive collateral circulation of the hand,11 RAO should be prevented in regard to the need for repeated catheterizations in the majority of patients with coronary artery disease. The purpose of this study is to carry out a prospective, large-cohort investigation into the incidence of RAO after coronary angiogram or percutaneous coronary intervention in a center with a high patient turnover and vast experience in radial access procedures.
Methods
Design and study population. Our study cohort included prospectively consecutive patients referred for coronary angiography and/or angioplasty using transradial access at our center in 2019. This prospective study of current treatments was purely observational. The inclusion criteria were as follows: coronary angiography/angioplasty via radial access, age >18 years, oral informed consent, and palpable radial pulse. The following exclusion criteria applied: patient under legal guardianship, unstable patients, or those presenting with arteriovenous fistula or Raynaud’s syndrome. The entire cardiology intervention team at the Groupe Hospitalier Mutualiste de Grenoble and the paramedical team were involved in this study, which started on January 1, 2019 and continued throughout the year.
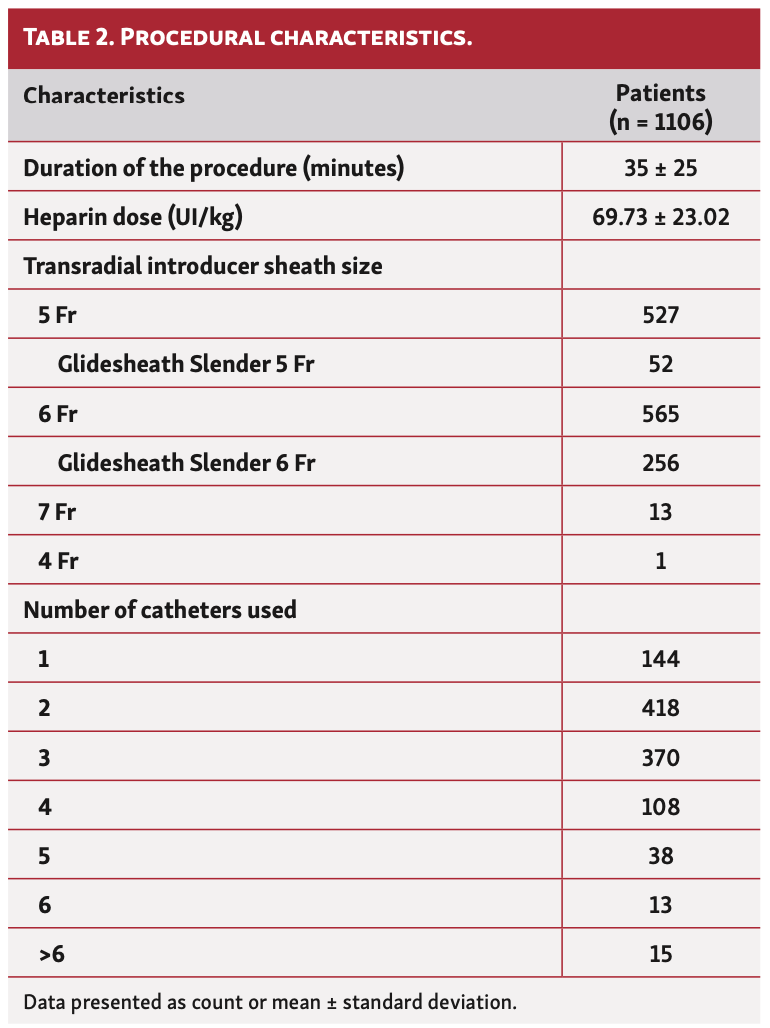
Transradial procedure. Each procedure was performed under local anesthesia with first application of a local anesthetic cream (EMLA) approximately 1 hour before, and then a local subcutaneous anesthesia (2% lidocaine). The puncture was a classic double-wall-through-and-through approach with a 20 gauge needle. Every patient received a bolus of intravenous heparin (dose left to operator’s preference) and a bolus of 5 mg diltiazem following insertion of a 7 cm introducer radial-dedicated Terumo sheath (5 Fr for diagnostic and 6 Fr or larger for angioplasty) over a 0.025˝ guidewire. Choice of catheter shape and number of catheters were left to the operator’s discretion.
Radial artery compression was achieved using the TR Band compression system (Terumo). Following immediate removal of the sheath at the end of the procedure, the bracelet was applied at the arterial puncture site and inflated to 15 mL with air. The cuff was then slightly deflated until minor bleeding was observed in order to purge the prethrombotic radial material, then reinflated by 2 mL, allowing patent radial artery flow. Each patient was transferred to the monitoring room, where patent hemostasis was performed by testing with a plethysmography signal in the thumb during manual compression of the ulnar artery. The cuff was deflated by 1 mL until a signal was obtained in the absence of bleeding or hematoma. The TR Band was gradually deflated by the nurse (generally 2 mL by 2 mL at H1, H2, H3, before being withdrawn at H4). A compressive dressing was then applied with instruction to elevate the hand slightly.
Study endpoints. The primary endpoint of the study was the incidence of early (within 24 hours of the procedure) RAO using Doppler ultrasound and ultrasonography. Ultrasound evaluations were recorded using a mobile vascular Doppler set (CONTEC) before and after compression of the ipsilateral ulnar artery. If RAO was suspected, an in-depth vascular Doppler ultrasound examination was performed (LOGIQ S7; GE Healthcare), studying 2-dimensional (2D), Doppler, and color Doppler parameters. The examination was routinely compared with the contralateral arm. RAO was defined according to the following criteria: no audible signal on the Doppler confirmed by Doppler ultrasound scan, revealing an absence of blood flow in the radial artery with thrombotic material confirmed with 2D ultrasonography.12 Each occlusion was checked again at 3 months.
Secondary study endpoints included presence of radial pulse, hematoma using the EASY hematoma scale,13 and access-site complications (ischemic hand, arteriovenous fistula, pseudoaneurysm). Demographic data, including age, sex, body mass index, risk factors, indications for coronary angiography or angioplasty, antiplatelet and anticoagulation treatments, were recorded. Procedural data, including sheath size, heparin dose, number of catheters, and procedural time, were also recorded.
Statistical analysis. Results are presented as mean ± standard deviation for continuous variables and as percentages for categorical data. Qualitative and quantitative covariates were compared using the Fisher's exact test and Wilcoxon test, respectively. RAO incidence was evaluated in univariate logistic regression analysis. We investigated predictive factors of RAO during follow-up for the entire patient population. Predictive factors for RAO were subsequently investigated. Comparisons were performed between the RAO group and the group without RAO. All covariates with a P-value <.05 were included in a multivariate logistic regression model to investigate independent predictors of RAO. All analyses were performed using R statistical software.
Results
Demographic data. Overall, a total of 1106 patients were enrolled over the 12-month investigation period. The demographics of the enrolled patients are outlined in Table 1. The data indicate an average age of 70 years. The population was divided as follows: 24.5% females and 75.5% males; body mass index of 28.1 kg/m2; 28.3% were diabetics; and mean creatinine clearance was 78.3 mL/min. Furthermore, 24.9% of patients had already undergone coronary angioplasty.
The reasons for seeking a medical consultation varied, but the following should be noted in particular: angina, 235 patients; silent ischemia, 466 patients; and acute coronary syndrome, 232 patients presenting a non-ST segment elevation myocardial infarction (NSTEMI), and 81 patients a ST-segment elevation myocardial infarction (STEMI). In terms of the overall cohort, 113 patients were taking vitamin K antagonist or direct oral anticoagulants.
Procedural characteristics. The procedural data recorded during the investigation are shown in Table 2. The duration of the procedure varied considerably, averaging 35 minutes. Similarly, the average heparin dose was 69.73 IU/kg. Technically, patent hemostasis could be achieved in 1091 patients (98.6%). Among those patients in whom patent hemostasis could not be performed, none presented with RAO.
Primary and secondary endpoints. Eleven RAOs were diagnosed (0.99%). Radial pulse was present in 1097 patients (99.2%); among those with RAO, 2 patients had a clinical radial pulse. A vascular echo-Doppler procedure was performed on each RAO for confirmation. Our study recorded a hematoma rate of 5.3% (59 hematomas), all of which were clinically irrelevant and none of which had a diameter >5 cm.
During follow-up on the 11 patients with RAO, 6 were confirmed at 3 months, 2 occlusions progressed favorably and spontaneously, 2 patients were lost to follow-up, and 1 female patient died prior to follow-up. None of the patients with RAO presented with hand ischemia clinical symptoms. With regard to hematomas, our study found a rate of 5.3% (59 hematomas); none of these hematomas had clinical consequences. One patient presented with an arteriovenous fistula post coronarography and required vascular surgery after failure of compression.
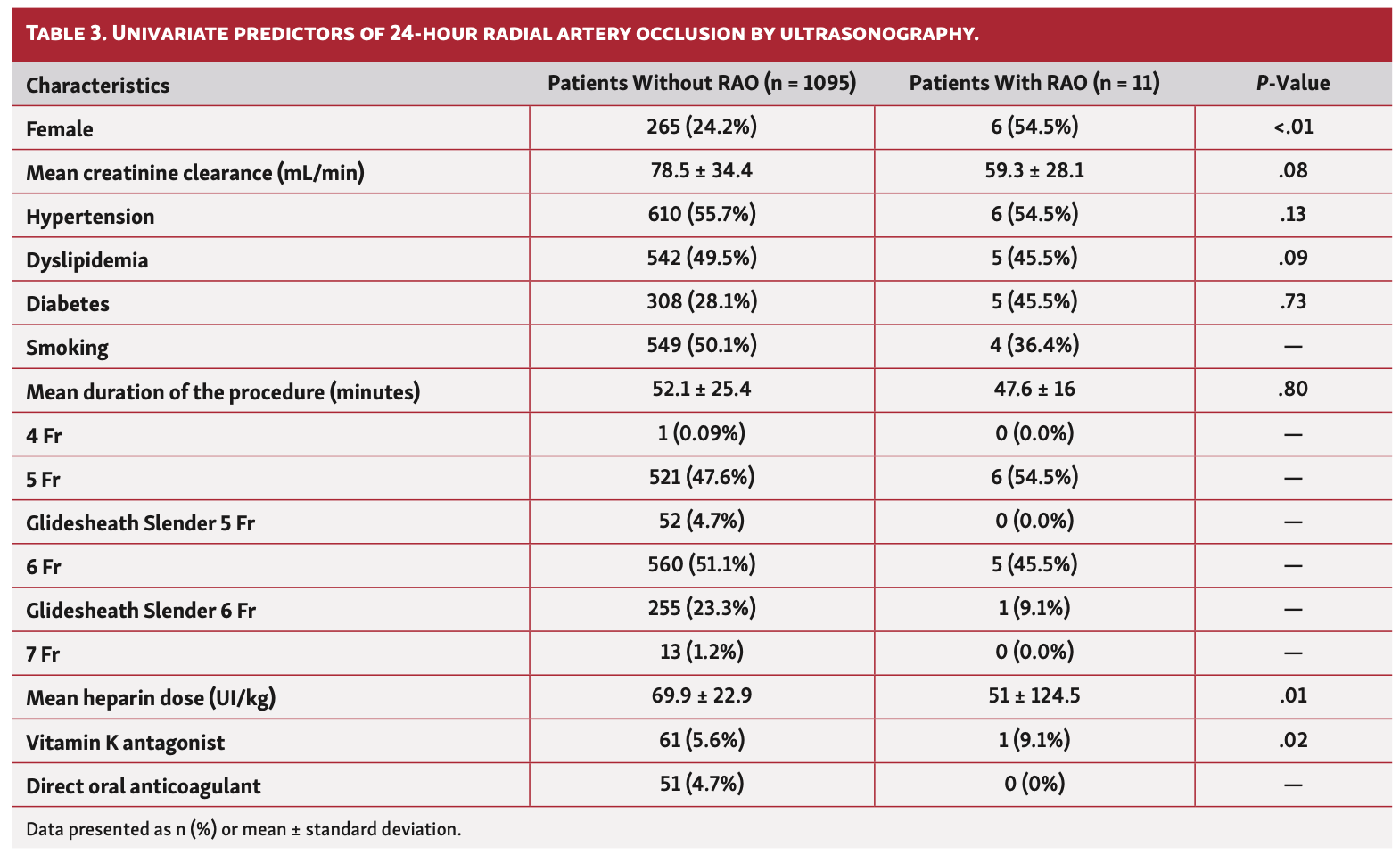
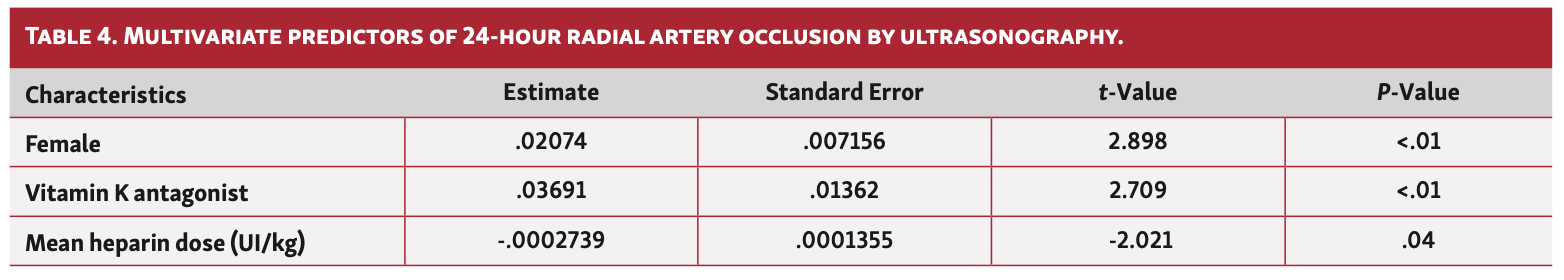
Univariate predictors of 24-hour RAO, including gender, creatinine clearance, cardiovascular risk factors, duration of the procedure, sheath size, mean heparin dose, treatment by vitamin K antagonist, direct oral anticoagulant, aspirin, and P2Y12 inhibitors, are shown in Table 3. Finally, we conducted a multivariate analysis following a logistics model to predict the risk of RAO using significant variables in univariate analysis (Table 4). The “estimate” column gives the value of the coefficient in the model (the value of the exponential constant is OR). OR is the exponential of the first column. With regard to the results and exercising caution given the very low rate of RAO, female gender was significantly associated with an increased risk of RAO in our study, such as treatment of VKA. Conversely, high doses of heparin protect against radial occlusion.
Discussion
Our study highlights a low rate of RAO following coronary angiography and/or angioplasty, with a rate of 0.99% in a large, single-center cohort of 1106 patients. The most consistent and interesting information is the fact that RAO is very rare in our prospective cohort. It should be remembered that the predictive factors of postprocedural RAO according to the literature are the size of the sheath,5,6,14 non-injection of heparin during the procedure,5,15 and the quality and duration of compression.9,16,16-20 This very low rate of RAO can be explained by several factors, such as practitioner experience, anticoagulation, and the use of low-profile sheaths. However, the more probable explanation is the fact that we prioritize patent hemostasis. Indeed, patent hemostasis is a major preoccupation after each procedure from a medical and paramedical perspective. Given the small patient population presenting with RAO, the predictive factors cannot be strongly evaluated statistically. Nevertheless, we will discuss each of the main predictive factors.
Patent flow hemostasis. Patent flow hemostasis has been shown to be a protective factor of RAO.10 In our study, patent hemostasis was routinely investigated in the monitoring room for each patient in our study. The concept of patent flow hemostasis21 by maintaining radial artery flow during its compression, has considerably reduced the risk of postprocedural RAO. Plethysmography analysis during compression gives an excellent indication of radial permeability throughout compression and reduced the RAO rate to 2%.22 In clinical routine, the first step is the correct positioning of the TR Band compression system; the sheath is then slowly withdrawn to reduce the risk of pain and therefore spasm. The TR Band is then slightly deflated until minor bleeding is observed, indicating removal of the prethrombotic material. The reverse-Barbeau test is subsequently performed in the monitoring room. Patent hemostasis is confirmed on detection of a plethysmography signal following manual compression of the ulnar cubital artery. The patent hemostasis achievement in the monitoring room shortly after the sheath removal, which allows an appropriate interval for any vasospastic reaction, plays a key role in our approach. In case of failure, the test should be repeated after 15 minutes. Edris et al have shown that the TR Band could be safely deflated significantly below the patent hemostasis point without bleeding by waiting 15 minutes after placement of the compression device.22 Shorter duration of hemostatic compression (2 hours) has been identified as an additional protective factor against RAO without increase in bleeding complications.23 However, this deflation must not be too rapid, as it may increase the risk of bleeding and therefore subsequently over-inflate the compression system.24 Compression of the ipsilateral ulnar artery during compression coupled with patent hemostasis has also had a significant impact in reducing the rate of RAO (0.9%).25,26 This calls for specific paramedical attention to detail and experience. The development of coronary angiography in an outpatient setting will reduce the compression time and therefore the rate of RAO in the future.
Radial artery catheterization. Radial artery catheterization may trigger an endothelial lesion and media dissection.27 A decrease in the diameter and lumen of the radial artery has also been noted during recurrent coronary angiography procedures.6,12,27,28 In fact, repeat catheterization triggers intimal hyperplasia with increased thickness, which can make subsequent puncture more difficult. Dahm et al demonstrated a reduction in the rate of RAO when using a 5 Fr catheter as opposed to a 6 Fr catheter.29 This difference was also noted in our study, but without any statistical relevance given the small number of events. The puncture site has been described by some operators as a predictive factor for RAO. It would seem that distal puncture, close to the radial styloid, might trigger the risk of RAO in addition to being more painful.30 The use of slender 5 Fr and 6 Fr sheaths may reduce the risk of mechanical microinjury in the radial artery and therefore RAO31 by reducing the external diameter while maintaining an adequate internal lumen and benefiting from the hydrophilic profile of the sheath. Another point is the use in our daily practice of a low-profile Terumo sheath (5 Fr for diagnostic and 6 Fr for angioplasty), first-line, so-called “pediatric,” measuring 7 cm in length, which allows a tapered transition zone, and probably a less aggressive radial catheterization.
Anticoagulation. Heparin anticoagulation is essential in preventing RAO. This point has now been accepted and is an integral part of our daily practice, with routine heparin bolus administration at the beginning of the examination followed by a supplement in the event of angioplasty. The prophylactic role of anticoagulation in this instance is dose-dependent; higher doses have been described by some authors as the most efficient protective factor against RAO.10 Our study corroborates these results with an average dose of 69.73 IU/kg. In this way, a multicenter, randomized study and the latest meta-analysis32 demonstrated a significant benefit of higher (100 IU/Kg) over standard heparin dose (50 IU/Kg) for prevention of RAO.33 In the event of a long procedure, activated clotting time (ACT) must be routinely administered in order to reduce the risk of catheter-induced thrombosis and RAO. The data from our study confirm the protective nature of heparin using univariate and multivariate analysis and encourage the use of high heparin dose. An interesting point in our study is that treatment by VKA could influence the RAO incidence (P<.01). This result can be explained by the low heparin dose received by these patients in order to prevent hemorrhagic risk complications. Considering these patients, we have to reconsider this practice and find a more adapted dose of heparin.
Radial artery compression. Radial artery compression is a key element of RAO following coronary angiography. Indeed, if the compression is too aggressive, the anterograde flow will decrease and the risk of thrombosis will increase.9 Compression cuffs were created for sole use in radial compression following coronary angiography. In 2008, the PROPHET study validated the hemostatic concept of the oximetry-guided HemoBand compression cuff (HemoBand) and recorded a decrease in the RAO rate,20 and the RACOMAP study also confirmed a reduction in the rate of RAO using the TR Band, with an incidence rate of 4.4%.34 The positioning of the cuff, which may seem trivial at the end of the procedure, therefore warrants full attention. It must not be positioned too low because this exposes the patient to the risk of hematoma upstream from the cuff and therefore essentially over-inflation of the latter. Pain on removal of the sheath is also part of the vasospastic component of the radial artery post procedure. Progressive, gradual withdrawal can therefore help to protect the radial artery and reduce the patient’s pain.
Follow-up. During patient follow-up, 6 occlusions were confirmed at 3 months and 2 occlusions progressed favorably and spontaneously. Previous studies also traditionally show a rate of spontaneous repermeabilization at 3 months.35 We have no experience at our center of a patient who had required percutaneous recanalization. The application of ulnar compression during the patent hemostasis technique has been shown to reduce RAO rates;25 it is possible that patients who present with early RAO might benefit from ulnar compression. This will probably require further investigation in order to preserve the radial artery capital in patients who may require further coronary angiograms in the future.
Study limitations. First, radial artery evaluation period on day 1 may be slightly premature. Second, this is a single-center study. Third, RAO was only measured directly after the procedure; nevertheless, it is known that RAO can occur either directly post procedure or in the weeks to months afterward. This work does, however, present uniform results from a center with many years of experience in radial access procedures. Results highlight the predictive factors for RAO, ie, high doses of heparin and patent hemostasis during compression. However, they have no substantial statistical value, given the low rate of RAO.
Conclusion
Radial access has reduced the incidence of vascular complications following coronary angiography and coronary angioplasty, but introduces the risk of RAO. According to our study, a very low rate of RAO (0.99%) can be achieved. There are many protective factors for RAO, including high anticoagulation doses, technical experience, as well as the use of low-profile sheaths. However, patent hemostasis is probably the most important deterrent to RAO. These excellent results are possible due to a collaboration between the medical and paramedical professionals.
From the 1Institut Cardio-Vasculaire, Groupe Hospitalier Mutualiste, Grenoble, France.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Monségu is a consultant for the Society Terumo. The remaining authors report no conflicts of interest regarding the content herein.
Final version accepted June 3, 2020.
Address for correspondence: Alain Rougé, MD, Institut Cardio-Vasculaire, Groupe Hospitalier Mutualiste, 38000 Grenoble, France. Email: a.rouge@ghm-grenoble.fr
- Pristipino C, Pelliccia F, Granatelli A, et al. Comparison of access-related bleeding complications in women versus men undergoing percutaneous coronary catheterization using the radial versus femoral artery. Am J Cardiol. 2007;99:1216-1221.
- Benamer H, Louvard Y, Sanmartin M, et al. A multicentre comparison of transradial and transfemoral approaches for coronary angiography and PTCA in obese patients: the TROP registry. EuroIntervention. 2007;3:327-332.
- Montalescot G, Ongen Z, Guindy R, et al. Predictors of outcome in patients undergoing PCI. Results of the RIVIERA study. Int J Cardiol. 2008;129:379-387.
- Gilchrist IC. Laissez-faire hemostasis and transradial injuries. Catheter Cardiovasc Interv. 2009;73:473-474.
- Zhou Y, Zhao Y, Cao Z, et al. Incidence and risk factors of acute radial artery occlusion following transradial percutaneous coronary intervention. Zhonghua Yi Xue Za Zhi. 2007;87:1531-1534.
- Nagai S, Abe S, Sato T, et al. Ultrasonic assessment of vascular complications in coronary angiography and angioplasty after transradial approach. Am J Cardiol. 1999;83:180-186.
- Petroglou D, Didagelos M, Chalikias G, et al. Manual versus mechanical compression of the radial artery after transradial coronary angiography: the MEMORY multicenter randomized trial. JACC Cardiovasc Interv. 2018;11:1050-1058.
- Stella PR, Kiemeneij F, Laarman GJ, et al. Incidence and outcome of radial artery occlusion following transradial artery coronary angioplasty. Cathet Cardiovasc Diagn. 1997;40:156-158.
- Sanmartin M, Gomez M, Rumoroso JR, et al. Interruption of blood flow during compression and radial artery occlusion after transradial catheterization. Catheter Cardiovasc Interv. 2007;70:185-189.
- Rashid M, Kwok CS, Pancholy S, et al. Radial artery occlusion after transradial interventions: a systematic review and meta-analysis. J Am Heart Assoc. 2016;5:e002686.
- van Leeuwen MAH, Hollander MR, van der Heijden DJ, et al. The ACRA anatomy study (assessment of disability after coronary procedures using radial access): a comprehensive anatomic and functional assessment of the vasculature of the hand and relation to outcome after transradial catheterization. Circ Cardiovasc Interv. 2017;10:e005753.
- Wakeyama T, Ogawa H, Iida H, et al. Intima-media thickening of the radial artery after transradial intervention. An intravascular ultrasound study. J Am Coll Cardiol. 2003;41:1109-1114.
- Bertrand OF, Larose E, Rodés-Cabau J, et al. Incidence, predictors, and clinical impact of bleeding after transradial coronary stenting and maximal antiplatelet therapy. Am Heart J. 2009;157:164-169.
- Yoo B-S, Yoon J, Ko J-Y, et al. Anatomical consideration of the radial artery for transradial coronary procedures: arterial diameter, branching anomaly and vessel tortuosity. Int J Cardiol. 2005;101:421-427.
- Spaulding C, Lefèvre T, Funck F, et al. Left radial approach for coronary angiography: results of a prospective study. Cathet Cardiovasc Diagn. 1996;39:365-370.
- Davis FM, Stewart JM. Radial artery cannulation. A prospective study in patients undergoing cardiothoracic surgery. Br J Anaesth. 1980;52:41-47.
- Bedford RF, Wollman H. Complications of percutaneous radial-artery cannulation: an objective prospective study in man. Anesthesiology. 1973;38:228-236.
- Slogoff S, Keats AS, Arlund C. On the safety of radial artery cannulation. Anesthesiology. 1983;59:42-47.
- Cubero JM, Lombardo J, Pedrosa C, et al. Radial compression guided by mean artery pressure versus standard compression with a pneumatic device (RACOMAP). Catheter Cardiovasc Interv. 2009;73:467-472.
- Pancholy S, Coppola J, Patel T, et al. Prevention of radial artery occlusion-patent hemostasis evaluation trial (PROPHET study): a randomized comparison of traditional versus patency documented hemostasis after transradial catheterization. Catheter Cardiovasc Interv. 2008;72:335-340.
- Pancholy SB, Bertrand OF, Patel T. Comparison of a priori versus provisional heparin therapy on radial artery occlusion after transradial coronary angiography and patent hemostasis (from the PHARAOH study). Am J Cardiol. 2012;110:173-176.
- Edris A, Gordin J, Sallam T, et al. Facilitated patent haemostasis after transradial catheterisation to reduce radial artery occlusion. EuroIntervention. 2015;11:765-771.
- Pancholy SB, Patel TM. Effect of duration of hemostatic compression on radial artery occlusion after transradial access. Catheter Cardiovasc Interv. 2012;79:78-81.
- Hromádka M, Bernat I, Seidlerová J, et al. Access-site bleeding and radial artery occlusion in transradial primary percutaneous coronary intervention: influence of adjunctive antiplatelet therapy. Coron Artery Dis. 2016;27:267-272.
- Pancholy SB, Bernat I, Bertrand OF, et al. Prevention of radial artery occlusion after transradial catheterization: the PROPHET-II randomized trial. JACC Cardiovasc Interv. 2016;9:1992-1999.
- Tian J, Chu Y-S, Sun J, et al. Ulnar artery compression: a feasible and effective approach to prevent the radial artery occlusion after coronary intervention. Chin Med J (Engl). 2015;128:795-798.
- Yonetsu T, Kakuta T, Lee T, et al. Assessment of acute injuries and chronic intimal thickening of the radial artery after transradial coronary intervention by optical coherence tomography. Eur Heart J. 2010;31:1608-1615.
- Edmundson A, Mann T. Nonocclusive radial artery injury resulting from transradial coronary interventions: radial artery IVUS. J Invasive Cardiol. 2005;17:528-531.
- Dahm JB, Vogelgesang D, Hummel A, et al. A randomized trial of 5 vs. 6 French transradial percutaneous coronary interventions. Catheter Cardiovasc Interv. 2002;57:172-176.
- Bi X-L, Fu X-H, Gu X-S, et al. Influence of puncture site on radial artery occlusion after transradial coronary intervention. Chin Med J (Engl). 2016;129:898-902.
- Aminian A, Saito S, Takahashi A, et al. Comparison of a new slender 6 fr sheath with a standard 5 fr sheath for transradial coronary angiography and intervention: RAP and BEAT (radial artery patency and bleeding, efficacy, adverse event), a randomised multicentre trial. EuroIntervention. 2017;13:e549-e556.
- Hahalis G, Aznaouridis K, Tsigkas G, et al. Radial artery and ulnar artery occlusions following coronary procedures and the impact of anticoagulation: ARTEMIS (radial and ulnar artery occlusion meta-analysis) systematic review and meta-analysis. J Am Heart Assoc. 2017;6:e005430.
- Hahalis GN, Leopoulou M, Tsigkas G, et al. Multicenter randomized evaluation of high versus standard heparin dose on incident radial arterial occlusion after transradial coronary angiography: the SPIRIT OF ARTEMIS study. JACC Cardiovasc Interv. 2018;11:2241-2250.
- Pancholy SB. Impact of two different hemostatic devices on radial artery outcomes after transradial catheterization. J Invasive Cardiol. 2009;21:101-104.
- Garg N, Madan BK, Khanna R, et al. Incidence and predictors of radial artery occlusion after transradial coronary angioplasty: Doppler-guided follow-up study. J Invasive Cardiol. 2015;27:106-112.