Mid-Term Results of Everolimus-Eluting Stent in a Japanese Population Compared With a US Randomized Cohort: SPIRIT III Japan Registry With Harmonization by Doing
Abstract: To address safety concerns with first-generation drug-eluting stents (DESs), the everolimus-eluting stent (EES) has been developed as a second-generation DES. The study aim was to: (1) demonstrate that use of the EES in Japanese patients is non-inferior to use of the paclitaxel-eluting stent (PES) in US patients; and (2) compare vessel response to the EES in Japanese vs US patients. Methods. The SPIRIT III Japan Registry, a prospective single-arm multicenter study was a part of the SPIRIT III global clinical program using harmonization by doing. The primary endpoint was in-segment late loss at 8 months, compared to US PES. Results. A total of 88 subjects were enrolled in the Japan EES group. Angiographic in-segment late loss was significantly less in Japan EES vs US PES (0.15 ± 0.34 mm vs 0.28 ± 0.48 mm, respectively; P=.0185; Pnon-inferior<.0001), while target vessel failure (TVF; 8.0% vs 9.9%) and major adverse cardiac events (MACE) at 9 months (5.7% vs 8.8%) were not significantly different between the 2 groups. No differences were observed between Japan and US EES populations in terms of late loss, TVF, or MACE. Neointimal volume and postprocedural incomplete stent apposition rate were lower in Japan EES vs US EES/PES. Conclusion. The SPIRIT III Japan Registry met the primary endpoint of lower late loss in the Japan EES group vs the US PES group, with comparable results for EES between the Japanese and US patients.
J INVASIVE CARDIOL 2012;24(9):444-450
Key words: drug-eluting stent, coronary artery disease, trials
____________________________________________________
The polymer-based, site-specific delivery of sirolimus (Cypher; Cordis Corporation) and paclitaxel (Taxus Express; Boston Scientific), developed as first-generation drug-eluting stents (DESs), has been shown to suppress neointimal hyperplasia after coronary stent implantation and to improve mid- to long-term efficacy compared with bare-metal stents (BMSs).1,2 However, the incidence of stent thrombosis is relatively increased compared with BMSs, likely due to delayed and incomplete endothelialization.3-5 To improve the safety concerns of first-generation DESs, everolimus-eluting stents (EESs; XIENCE V, Abbott Vascular) have been developed as second-generation DESs.6,7
Although a number of trials using first- and second-generation DESs showed consistent results of neointimal suppression compared with BMSs, the clinical impact of different ethnicities and/or different procedures has not yet been investigated. In addition, the harmonization by doing (HBD) program, which is a unique collaboration among regulatory authorities, industry manufacturers, and academic clinicians to focus on improvement of quality and approval timelines, was introduced to evaluate new devices; however, it has not yet been fully scientifically evaluated. The aim of this study was to demonstrate: (1) non-inferiority of EESs in Japanese patients compared to the US paclitaxel-eluting stent (PES) arm with the HBD; and (2) vessel response of EES in the two different nationalities. This is the first report comparing Japan and US subjects with either EES or PES using the same inclusion and exclusion criteria.
Methods
 Trial overview and study population. The SPIRIT III Japan Registry (Japan EES), a prospective, single-arm, Japanese multicenter study was conducted as a part of the SPIRIT III global clinical trial (Figure 1). In parallel with the Japan Registry, the US randomized control trial (RCT) (1,002 subjects, 2:1 randomization of the XIENCE V and the Taxus)8 and the 4.0 mm Registry (80 subjects)9 were also conducted. The Japan Registry was designed to evaluate the efficacy and safety of treatment with EES for Japanese subjects with de novo native coronary artery lesions (single lesion in a single vessel or two lesions in two different vessels).
Trial overview and study population. The SPIRIT III Japan Registry (Japan EES), a prospective, single-arm, Japanese multicenter study was conducted as a part of the SPIRIT III global clinical trial (Figure 1). In parallel with the Japan Registry, the US randomized control trial (RCT) (1,002 subjects, 2:1 randomization of the XIENCE V and the Taxus)8 and the 4.0 mm Registry (80 subjects)9 were also conducted. The Japan Registry was designed to evaluate the efficacy and safety of treatment with EES for Japanese subjects with de novo native coronary artery lesions (single lesion in a single vessel or two lesions in two different vessels).
In these studies, the same inclusion and exclusion criteria were applied except for reference diameter (Japan: 2.5-4.25 mm; US RCT: 2.5-3.75 mm). Key angiographic inclusion criteria included a maximum of two de novo native coronary artery lesions, each in a different epicardial vessel; reference diameter of ≥2.5 mm and ≤4.25 mm; lesion length ≤28 mm by visual estimation; percent diameter stenosis (%DS) of ≥50% and <100%. Key angiographic exclusion criteria included aorto-ostial location, left main location, excessive tortuosity, extreme angulation (≥90°), heavy calcification, target vessel containing thrombus, and other significant lesions (>40% diameter stenosis) in the target vessel or side branch for which intervention was required within 9 months. If two target lesions were treated, each of these lesions had to meet all angiographic inclusion/exclusion criteria.
The study was approved by the institutional review board at each enrolling site, and eligible patients signed written informed consent before the interventional procedure.
Anti-platelet drug therapy regimen. In this Japan EES group, only subjects tolerant to ticlopidine were enrolled. Preprocedural administration of ticlopidine 200 mg daily for at least 3 days prior to the index procedure was prescribed. Loading dose of aspirin was to be at least 300 mg. Subjects enrolled into the Japan EES were required to receive oral ticlopidine 200 mg daily for at least 90 days. A daily dose of oral aspirin, 80 mg or more, was also to be administered for 5 years. In the US RCT, the protocol recommended that patients receive aspirin (≥80 mg daily) indefinitely and clopidogrel 75 mg daily for a minimum of 6 months.8
Data management and core laboratory. All data were collected on electronic case report forms (eCRF) (InForm; Phase Forward). Endpoint events were adjudicated by a Clinical Events Committee (Duke Cardiovascular Research Institute in Durham, North Carolina). Stent thromboses as defined by the Academic Research Consortium were independently adjudicated by the Harvard Clinical Research Institute in Boston, Massachusetts.
Coronary angiograms performed at baseline and follow-up (240 ± 28 days after the procedure) were reviewed by an independent angiographic core laboratory (Cardiovascular Research Foundation in New York, New York) using a standard manner.10,11 Similarly, IVUS images were examined by an independent core laboratory (Cardiovascular Core Analysis Laboratory, Stanford University in Stanford, California) and standard parameters were achieved.12,13
Study endpoint. The primary endpoint was in-segment late loss at 8 months, which was the same as the SPIRIT III RCT. According to the unique study design arising from the HBD initiative, the primary endpoint was to be tested against the control group, the PES angiographic subgroup in the SPIRIT III RCT (Figure 1). To avoid inter-lesion clustering of restenosis in patients receiving stents for multiple lesions (which would have required correction with multilevel generalized estimating equations), the protocol specified that in patients with 2 lesions treated, a single lesion (the analysis lesion) would be randomly selected for analysis of late loss.
The major secondary endpoints were as follows: (1) angiographic endpoint: in-stent late loss, in-stent and in-segment %DS at 8 months; (2) IVUS endpoint: percent neointimal obstruction and late incomplete stent apposition at 8 months; and (3) clinical endpoint: ischemic-driven target vessel failure (TVF: cardiac death, myocardial infarction, ischemic driven target lesion revascularization [TLR], or ischemic driven non-target vessel revascularization [TVR]), MACE (cardiac death, myocardial infarction, or TLR), TLR, and stent thrombosis at 9-month follow-up exam.
Statistical methods. For the Japan EES group, the overall sample size of 88 subjects, assuming 10% drop-outs, was calculated based on the primary endpoint of in-segment late loss at 240 days. The sample size would have a 92% statistical power with a non-inferiority delta of 0.195 mm, true in-segment late loss of 0.24 ± 0.47 mm in both treatment arms with an overall 5% alpha (one-sided), and 10% drop-outs. Due to the inclusion of dual-lesion treatment, primary endpoint hypothesis testing was based on the intent-to-treat population using the ‘analysis lesion’ (target lesion in single-vessel subjects and a randomly selected lesion in dual-vessel subjects). The primary endpoint was to be tested against the control group, the PES angiographic subgroup in the SPIRIT III RCT.
Categorical variables were compared by Fisher’s exact test. Continuous variables are expressed as mean ± standard deviation and were compared by t-test. To take the oculostenotic reflex into consideration, description analysis was performed to compare the results with those of subjects in the US angiographic arms. Since the purpose of this study was to compare Japan EES vs US PES and Japan EES vs US EES (Figure 1), ANOVA was not performed. Multiple linear regression analysis was performed to detect factors that influenced late loss and neointimal hyperplasia.
A P-value of .05 was established as the level of statistical significance for all tests. All analyses were performed with SAS software (SAS Institute).
Results
Study population. A total of 88 subjects were enrolled into the Japan EES at 12 Japanese sites, as scheduled. Of these, 87 subjects (98.9%) completed the 9-month follow-up visit. A reason for termination prior to the 9-month follow-up visit was death, but we included this in the 9-month analysis.
 Baseline patient characteristics. Baseline patient characteristics are summarized in Table 1. In the Japan EES, 48.9% of subjects (43/88) had hypercholesterolemia requiring medication, which was lower than the US EES and PES arms (74.2% and 69.0%, respectively). Other coronary risk factors in the Japan EES group were comparable to those in the US arms.
Baseline patient characteristics. Baseline patient characteristics are summarized in Table 1. In the Japan EES, 48.9% of subjects (43/88) had hypercholesterolemia requiring medication, which was lower than the US EES and PES arms (74.2% and 69.0%, respectively). Other coronary risk factors in the Japan EES group were comparable to those in the US arms.
There were significant differences in height, weight, and obesity status between the Japan and US EES/PES arms. The mean body mass index (BMI) was 23.8 kg/m2 in the Japan EES. Only 2.3% (2/88) in the Japan EES population were obese with BMI ≥30, while around 50% in the US arm were obese.
 Baseline lesion and procedural characteristics. Lesion characteristics with unselected lesions were comparable between the Japan EES and US arms (Table 2). Of the 88 subjects in the Japan EES arm analyzed at baseline, 15.9% (14/88) had dual lesions, and the total number of target lesions was 102. On the other hand, 11.2% (51/445) in the US EES arm and 17.0% (32/188) in the US PES arm had dual lesions, resulting in the total number of lesions as 496 and 188, respectively. Although the Japan EES group tended to have a higher rate of type B2/C lesions compared with the US arms, preprocedure mean lesion length, reference diameter, minimum lumen diameter and %DS were comparable among the 3 treatment arms.
Baseline lesion and procedural characteristics. Lesion characteristics with unselected lesions were comparable between the Japan EES and US arms (Table 2). Of the 88 subjects in the Japan EES arm analyzed at baseline, 15.9% (14/88) had dual lesions, and the total number of target lesions was 102. On the other hand, 11.2% (51/445) in the US EES arm and 17.0% (32/188) in the US PES arm had dual lesions, resulting in the total number of lesions as 496 and 188, respectively. Although the Japan EES group tended to have a higher rate of type B2/C lesions compared with the US arms, preprocedure mean lesion length, reference diameter, minimum lumen diameter and %DS were comparable among the 3 treatment arms.
 Angiographic and IVUS results at 8 months. The primary endpoint (in-segment late loss at 8 months) with “selected” lesion was 0.15 ± 0.34 mm (83 analysis lesions) in the Japan EES and 0.28 ± 0.48 mm in the US PES (134 analysis lesions), with a 46% reduction (P=.0185). The non-inferior P-value with 0.195 mm non-inferior margin was <.0001, which demonstrated non-inferiority of the Japan EES to the US PES group. As shown in Table 3, for the “unselected” population including cases of multiple lesions, in-stent and in-segment late loss
Angiographic and IVUS results at 8 months. The primary endpoint (in-segment late loss at 8 months) with “selected” lesion was 0.15 ± 0.34 mm (83 analysis lesions) in the Japan EES and 0.28 ± 0.48 mm in the US PES (134 analysis lesions), with a 46% reduction (P=.0185). The non-inferior P-value with 0.195 mm non-inferior margin was <.0001, which demonstrated non-inferiority of the Japan EES to the US PES group. As shown in Table 3, for the “unselected” population including cases of multiple lesions, in-stent and in-segment late loss 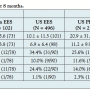 were significantly lower in the Japan EES compared with the US PES group. However, there were no significant differences between the Japan EES and US EES arms. As for edge stenosis, no cases occurred in the Japan Registry, while 2.3% in US EES and 3.2% in US PES were observed.
were significantly lower in the Japan EES compared with the US PES group. However, there were no significant differences between the Japan EES and US EES arms. As for edge stenosis, no cases occurred in the Japan Registry, while 2.3% in US EES and 3.2% in US PES were observed.
Neointimal volume was significantly lower in the Japan EES compared with the US EES and US PES arms. Postprocedural incomplete stent apposition (ISA) was observed with significantly higher frequency in both the US EES and US PES arms compared with the Japan EES group. Late ISA was observed in 1 case in each group (Table 4).
 Clinical outcomes at 9 months. The TLR and TVF rate in the Japan EES group were comparable to the US EES arm and the US PES arm. The MACE rate at 9 months was also comparable among the 3 groups (Japan EES, 5.7%; US EES,5.5%; US PES, 8.8%). The Kaplan-Meier curves for TLR, TVF, and MACE are shown in Figure 2. There was no occurrence of stent thrombosis (protocol/ARC) in the Japan EES group at the 9-month follow-up exam, while 1 case was observed in the US EES group and 2 cases in the US PES group (Table 5).
Clinical outcomes at 9 months. The TLR and TVF rate in the Japan EES group were comparable to the US EES arm and the US PES arm. The MACE rate at 9 months was also comparable among the 3 groups (Japan EES, 5.7%; US EES,5.5%; US PES, 8.8%). The Kaplan-Meier curves for TLR, TVF, and MACE are shown in Figure 2. There was no occurrence of stent thrombosis (protocol/ARC) in the Japan EES group at the 9-month follow-up exam, while 1 case was observed in the US EES group and 2 cases in the US PES group (Table 5).
 Multiple linear regression analysis. Multiple linear regression analysis, including different baseline characteristics such as age, hypertension, angina type, target lesion, lesion length, frequency of postdilatation, and patient nationality, confirmed the Japan arm was independently associated with in-segment late loss in comparison with US PES (standardized coefficient estimate, 0.210; 95% confidence interval [CI], 0.040-0.380; P=.016). Similarly, the Japan arm was independently associated with neointimal obstruction in comparison with US EES and US PES (vs US EES: standardized coefficient estimate, 3.851; 95% CI,2.157-5.544; P<.001; and vs US PES: standardized coefficient estimate, 13.566; 95% CI, 9.048-18.084; P<.001).
Multiple linear regression analysis. Multiple linear regression analysis, including different baseline characteristics such as age, hypertension, angina type, target lesion, lesion length, frequency of postdilatation, and patient nationality, confirmed the Japan arm was independently associated with in-segment late loss in comparison with US PES (standardized coefficient estimate, 0.210; 95% confidence interval [CI], 0.040-0.380; P=.016). Similarly, the Japan arm was independently associated with neointimal obstruction in comparison with US EES and US PES (vs US EES: standardized coefficient estimate, 3.851; 95% CI,2.157-5.544; P<.001; and vs US PES: standardized coefficient estimate, 13.566; 95% CI, 9.048-18.084; P<.001).
Discussion
The main findings of this registry are as follows: (1) there were some differences in baseline characteristics and acute results between Japan and US populations; (2) the SPIRIT III Japan trial has met the primary endpoint in that angiographic late loss was significantly lower in the Japan EES group compared with the US PES group; (3) TVF and MACE were comparable between Japan EES and US PES; and (4) no significant differences were observed between the Japan and US EES groups in terms of angiographic and clinical outcomes at 9 months.
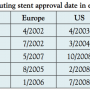 Trial design. The SPIRIT III was intended for approval of the XIENCE V everolimus-eluting coronary stent system in the United States and Japan. The SPIRIT III Japan non-randomized arm (the Japan Registry) was conducted as a part of the SPIRIT III global clinical trial using HBD. The HBD program represents a unique collaboration among regulatory authorities, industry manufacturers, and academic clinicians to focus on improvement of the quality and approval timelines, thereby lowering the cost of new device evaluations. Two DES trials, ENDEAVOR Japan and SPIRIT III, have been conducted using this unique method for Japanese approval. To save cost and time for enrolling patients, both trials were a single-arm registry, sharing the control group from the pivotal RCT. In addition, identical inclusion and exclusion criteria, endpoints, and core laboratories were used in the SPIRIT III trial. In this trial, the PES arm of the US RCT was used as the hypothesis control group for the Japan Registry as well as the direct control group for the US RCT. However, several limitations of this unique system arise when the HBD has been used in clinical trials. Even though sharing a control group to facilitate a more rapid approval, the mean time for Japanese approval of Endeavor (14 months) and Xience V (18 months) could not be shortened compared with those of the first generation of DESs (11 months in Cypher and 36 months in Taxus express) (Table 6). One of the main issues may be that regulatory requirements are changing year-by-year based on different social backgrounds of each time period. Moreover, although the same inclusion and exclusion criteria were used for both the Japanese and US arms, some differences were observed in baseline characteristics due to different nationality. In addition, different health insurance coverage/health care system may cause another bias. Different available drugs between Japan and the US may cause different prescriptions. In fact, at the time of SPIRIT III, since clopidogrel was not yet approved in Japan, ticlopidine was prescribed. These differences may affect interpretation of the results despite statistical methods such as multivariate analysis being performed to adjust for different patient backgrounds.
Trial design. The SPIRIT III was intended for approval of the XIENCE V everolimus-eluting coronary stent system in the United States and Japan. The SPIRIT III Japan non-randomized arm (the Japan Registry) was conducted as a part of the SPIRIT III global clinical trial using HBD. The HBD program represents a unique collaboration among regulatory authorities, industry manufacturers, and academic clinicians to focus on improvement of the quality and approval timelines, thereby lowering the cost of new device evaluations. Two DES trials, ENDEAVOR Japan and SPIRIT III, have been conducted using this unique method for Japanese approval. To save cost and time for enrolling patients, both trials were a single-arm registry, sharing the control group from the pivotal RCT. In addition, identical inclusion and exclusion criteria, endpoints, and core laboratories were used in the SPIRIT III trial. In this trial, the PES arm of the US RCT was used as the hypothesis control group for the Japan Registry as well as the direct control group for the US RCT. However, several limitations of this unique system arise when the HBD has been used in clinical trials. Even though sharing a control group to facilitate a more rapid approval, the mean time for Japanese approval of Endeavor (14 months) and Xience V (18 months) could not be shortened compared with those of the first generation of DESs (11 months in Cypher and 36 months in Taxus express) (Table 6). One of the main issues may be that regulatory requirements are changing year-by-year based on different social backgrounds of each time period. Moreover, although the same inclusion and exclusion criteria were used for both the Japanese and US arms, some differences were observed in baseline characteristics due to different nationality. In addition, different health insurance coverage/health care system may cause another bias. Different available drugs between Japan and the US may cause different prescriptions. In fact, at the time of SPIRIT III, since clopidogrel was not yet approved in Japan, ticlopidine was prescribed. These differences may affect interpretation of the results despite statistical methods such as multivariate analysis being performed to adjust for different patient backgrounds.
With its unique design, HBD allows sharing information as much as possible during the study period, thereby offering possibilities to reduce the timetable for the approval process. Although current approval processes using HBD may not work completely, this experience represents a first step toward global investigations not only between Japan and the US, but between Japan and the entire world. Finally, a more rapid introduction of new devices to the clinical setting may be of benefit to patient care. It is hoped that this study platform and design is recognized as a secure method by which the process for medical device approval would be expedited in a safe and efficacious manner.
Differences between Japan and US population. The SPIRIT III trial used the same inclusion/exclusion criteria for both Japanese and US patients, yet there were some differences in their clinical characteristics. In terms of patient demographics, the Japan arm included patients with higher age, lower incidence of hypercholesterolemia, and lower BMI, mainly due to differences in race/nationality and/or lifestyle. Regarding lesion characteristics, there were no significant differences among the 3 groups, indicating that differing patient demographics did not affect lesion characteristics. However, regarding procedural characteristics, significantly higher balloon pressures and higher frequency of postdilatation were observed in the Japanese cohort. These procedural differences may be related to acute results, such as the frequency of baseline ISA, suggesting that a higher frequency of postdilation contributed to the lower incidence of ISA in the Japan arm rather than the stent design and/or material. The more frequent use of postdilatation and other technical differences, such as higher maximum balloon pressure and/or appropriate balloon size, may have contributed to the achievement of better intravascular ultrasound (IVUS) outcomes in the Japan arm.
Late loss and clinical events. A variety of anti-proliferative agents mounted on stents have been tested; however, only sirolimus, paclitaxel, zotarolimus, and everolimus have been proven safe and effective in large, multicenter, randomized clinical trials. The overall effect of DES use is neointimal suppression, which is described as late loss by angiography and neointimal obstruction by IVUS. Previous reports have demonstrated in-stent late loss of 0.77-1.03 mm in BMSs,1,2,14-17 0.15-0.20 mm in sirolimus-eluting stents (SESs),1,14,18 0.30-0.52 mm in PESs,2,15,16,19 0.60-0.67 mm in Endeavor zotarolimus-eluting stents (ZESs),17-19 and 0.10-0.11 mm in EESs.6,7 The Japan XIENCE V trial showed an in-stent late loss of 0.10 mm, which was comparable to previously published data from SES as well as EES trials.
The significantly lower level of late loss with EES and SES translated into a modest level of TLR (2.3% in Japan EES, 3.2% in US EES at 9 months). On the other hand, ZES with a modest level of late loss reported a TLR rate of 4.1%-6.3%.17-19 These TLR differences were minimal compared with the difference in late loss. Mauri et al previously described the curvilinear relationship between late lumen loss and angiographic binary restenosis, and predicted a restenosis rate.20 Another report showed late loss can reliably estimate TLR rate for DESs and BMSs; thus, angiographic measurements were suitable for surrogate markers for clinical stent efficacy.21 However, evaluation of restenosis from late loss is more complex, especially in studies with a small sample size coupled with a relatively low occurrence of negative clinical events. In addition, since late loss did not show a normal distribution curve, difference of late loss would not be directly associated with clinical events.
To evaluate each stent’s efficacy, lesion to lesion variability should be considered.22 In a statistical distribution, late loss with BMSs showed a near Gaussian curve, and the mean value represents the overall biological response to the stent and the width of the distribution indicates lesion to lesion variability. Restenosis corresponds to the right tail end of the distribution curve above a threshold of ischemia. However, since significant biological modifications were applied to currently available DESs, their distribution showed a markedly skewed shift to the left, with variable shapes of the tail ends. Therefore, for the evaluation of DES efficacy, it may be insufficient to merely compare the mean value for each DES, since currently available DESs will have some cases at the right tail end of the distribution curve, regardless of DES type. Thus, the assessment of right tail end is also important to better understand each individual stent’s performance.
Study limitations. There were several limitations in this study. First, the primary endpoint of this study was to compare an angiographic surrogate endpoint, but the sample size did not enable adequate statistical power to examine differences of clinical endpoints. Second, even though this clinical trial used the same inclusion/exclusion criteria, the Japan Registry was a single-arm registry. Therefore, the identity of the treatment stent was known to the operator, which could introduce bias in procedural outcomes and the performance of repeat revascularization. Third, patient background including procedural characteristics was different between the Japan and the US. Although we adjusted for these differences using multivariate analysis to offset any bias, this is a limitation of the trial design (HBD). Furthermore, this trial was a non-inferiority test with a small population, which may cause issues such as choice of active control or selection of non-inferiority margin. Fourth, since clopidogrel was not approved in Japan, anti-platelet regimens were different between the Japanese and US populations. These differences may affect clinical outcomes. Fifth, the results from this clinical trial are specific to the patient population studied, with a relatively small number, and may not be generalized to the much broader population of patients with more complex lesions.
Conclusion
The SPIRIT III Japan Registry met the primary endpoint of non-inferior late loss in the Japan EES group compared to the US PES group, with comparable results for EESs between the Japanese and US cohorts. Procedural factors, such as the more frequent postdilatation and higher maximum balloon pressure and/or appropriate balloon sizing may have contributed to the achievement of better IVUS outcomes in Japanese patients. However, in this study, clinical events including MACE and TVF were comparable among all 3 groups.
References
- Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349(14):1315-1323.
- Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350(3):221-231.
- Pfisterer M, Brunner-La Rocca HP, Buser PT, et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol. 2006;48(12):2584-2591.
- Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48(1):193-202.
- Shite J. Delayed neointimalization on drug-eluting stents — speculation from optical coherence tomography. Circ J. 2009;73(12):2210-2211.
- Serruys PW, Ong AT, Piek JJ, et al. A randomized comparison of a durable polymer everolimus-eluting stent with a bare metal coronary stent: The SPIRIT first trial. EuroIntervention. 2005;1(1):58-65.
- Serruys PW, Ruygrok P, Neuzner J, et al. A randomised comparison of an everolimus-eluting coronary stent with a paclitaxel-eluting coronary stent: the SPIRIT II trial. EuroIntervention. 2006;2(3):286-294.
- Stone GW, Midei M, Newman W, et al. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA. 2008;299(16):1903-1913.
- Gordon PC, Applegate RJ, Hermiller JB, et al. Clinical and angiographic outcomes with an everolimus-eluting stent in large coronary arteries: the SPIRIT III 4.0 mm registry. Catheter Cardiovasc Interv. 2010;75(2):179-186.
- Ellis SG, Vandormael MG, Cowley MJ, et al. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Implications for patient selection. Multivessel Angioplasty Prognosis Study Group. Circulation. 1990;82(4):1193-1202.
- van der Zwet PM, Reiber JH. A new approach for the quantification of complex lesion morphology: the gradient field transform; basic principles and validation results. J Am Coll Cardiol. 1994;24(1):216-224.
- Sonoda S, Morino Y, Ako J, et al. Impact of final stent dimensions on long-term results following sirolimus-eluting stent implantation: serial intravascular ultrasound analysis from the sirius trial. J Am Coll Cardiol. 2004;43(11):1959-1963.
- Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37(5):1478-1492.
- Schofer J, Schluter M, Gershlick AH, et al. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomised controlled trial (E-SIRIUS). Lancet. 2003;362(9390):1093-1099.
- Colombo A, Drzewiecki J, Banning A, et al. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation. 2003;108(7):788-794.
- Stone GW, Ellis SG, Cannon L, et al. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA. 2005;294(10):1215-1223.
- Fajadet J, Wijns W, Laarman GJ, et al. Randomized, double-blind, multicenter study of the Endeavor zotarolimus-eluting phosphorylcholine-encapsulated stent for treatment of native coronary artery lesions: clinical and angiographic results of the ENDEAVOR II trial. Circulation. 2006;114(8):798-806.
- Kandzari DE, Leon MB, Popma JJ, et al. Comparison of zotarolimus-eluting and sirolimus-eluting stents in patients with native coronary artery disease: a randomized controlled trial. J Am Coll Cardiol. 2006;48(12):2440-2447.
- Leon MB, Mauri L, Popma JJ, et al. A randomized comparison of the ENDEAVOR zotarolimus-eluting stent versus the TAXUS paclitaxel-eluting stent in de novo native coronary lesions 12-month outcomes from the ENDEAVOR IV trial. J Am Coll Cardiol. 2010;55(6):543-554.
- Mauri L, Orav EJ, O’Malley AJ, et al. Relationship of late loss in lumen diameter to coronary restenosis in sirolimus-eluting stents. Circulation. 2005;111(3):321-327.
- Pocock SJ, Lansky AJ, Mehran R, et al. Angiographic surrogate end points in drug-eluting stent trials: a systematic evaluation based on individual patient data from 11 randomized, controlled trials. J Am Coll Cardiol. 2008;51(1):23-32.
- Honda Y. Drug-eluting stents. Insights from invasive imaging technologies. Circ J. 2009;73(8):1371-1380.
____________________________________________________
From 1ShonanKamakura General Hospital, Kamakura, Japan, 2Kyoto Katsura Hospital, Kyoto, Japan, 3Sakurabashi Watanabe Hospital, Osaka, Japan, 4Toho University Ohashi Medical Center, Tokyo, Japan, 5Teikyo University Hospital, Tokyo, Japan, 6Nagoya Daini Red Cross Hospital, Nagoya, Japan, 7Kawasaki Social Insurance Hospital, Kawasaki, Japan, 8Kyoto Second Red Cross Hospital, Kyoto, Japan, 9National Cerebral and Cardiovascular Center, Suita, Japan, 10Kurashiki Central Hospital, Kurashiki, Japan, 11Kyoto University Hospital, Kyoto, Japan, 12Hokkaido Social Insurance Hospital, Sapporo, Japan, 13Abbott Vascular Japan, Tokyo, Japan, 14Yale University School of Medicine, New Haven, Connecticut, 15Stanford University, Stanford, California, and 16Abbott Vascular, Santa Clara, California.
Funding: This study was supported by Abbott Vascular, Santa Clara, California.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. K. Saito is a consultant for Boston Scientific Japan. He reports speaker honoraria from Abbott Vascular Japan and Boston Scientific Japan. Dr Nakamura reports speaker honoraria from Abbott Vascular Japan and Boston Scientific Japan. Dr Fujii reports speaker honoraria from Abbott Vascular Japan. Dr Isshiki is a consultant for Abbott Vascular Japan, and reports speaker honoraria from Abbott Vascular Japan and Boston Scientific Japan. Dr Mitsudo is a consultant for Abbott Vascular Japan. Dr Kimura is a consultant for Abbott Vascular Japan. Dr Igarashi is a consultant for Abbott Vascular Japan. Dr Saito is employed by Abbott Vascular Japan. Dr Stone is a consultant for Abbott Vascular and reports speaker honoraria from Abbott Vascular and Boston Scientific. Dr Fitzgerald reports a consulting agreement with and research support from Abbott Vascular. Dr Sudhir is employed by Abbott Vascular.
Manuscript submitted January 16, 2012, provisional acceptance given February 22, 2012, final version accepted April 17, 2012.
Address for correspondence: Shigeru Saito, MD, Director, Division of Cardiology and Catheterization Laboratories, ShonanKamakura General Hospital, 1370-1 Okamoto, Kamakura, Japan 247-8533. Email: transradial@kamakuraheart.org
















