LAST (Local Anesthetic Systemic Toxicity) But Not Least: Systemic Lidocaine Toxicity During Cardiac Intervention
ABSTRACT: Lidocaine is the most common medication used for local anesthesia in cardiac procedures. Sometimes, a higher dose of lidocaine is used to improve the patient’s comfort, especially in device implantation or complex interventional procedures requiring several sheath insertions for access. We describe a patient with idiopathic cardiomyopathy who underwent implantable cardioverter defibrillator implantation for primary prevention and developed local anesthetic systemic toxicity (LAST) associated with lidocaine use. Multiple susceptible factors leading to lidocaine toxicity found in this case are common in patients with advanced heart failure. This case emphasizes the importance of dose adjustment of local anesthetic agents in individual patients, especially those with advanced heart failure who undergo cardiovascular procedures. The risk factors, preventive measures, and therapeutic approaches to manage this type of complication are discussed in detail.
J INVASIVE CARDIOL 2014;26(1):E13-E15
Key words: heart failure, lidocaine toxicity
________________________
Lidocaine is the most common medication used for local anesthesia in cardiac procedures. Sometimes, a higher dose of lidocaine is used to improve the patient’s comfort, especially in device implantation or complex interventional procedures requiring several sheath insertions for access. Complex cardiovascular procedures or device implantation using local anesthesia with mild to moderate conscious sedation has been widely practiced to facilitate these procedures and to avoid general anesthesia related cost and complications.1,2 Herein, we described a 56-year-old female with non-ischemic cardiomyopathy (NICM) who received an implantable cardioverter defibrillator (ICD) for primary prevention and developed local anesthetic systemic toxicity (LAST) due to subcutaneous injection with lidocaine during the procedure.
Case Report. A 56-year-old African-American female with NICM, hypertension, and diabetes was referred for evaluation of ICD implantation for primary prevention. The patient was in New York Heart Association class III. Her medication regimen included carvedilol 6.25 mg twice daily, candesartan 16 mg daily, hydralazine 25 mg three times daily, and isosorbide dinitrate 10 mg three times daily. Preoperative physical examination revealed blood pressure of 126/81 mm Hg, heart rate of 70 beats per minute, weight of 56.4 kg, elevated jugular venous pressure of 12 cm H2O along with hepatojugular venous reflux, and pansystolic murmur at left lower sternal border and apex without audible gallops. Laboratory investigation revealed sodium of 136 mmol/L, creatinine of 0.99 mg/dL, hemoglobin of 14.5 g/dL, albumin of 1.9 g/dL, total protein of 3.8 g/dL, alkaline phosphatase of 248 units/L (normal, 38-126 units/L) with normal transaminase enzyme and gamma-glutamyl transpeptidate was 209 unit/L (normal, 12-43 units/L). Her echocardiogram showed severe biventricular dysfunction with left ventricular ejection fraction of 10%-15% and right ventricular systolic pressure of 53 mm Hg.
In this setting, she was transferred for single-chamber ICD implantation. The procedure was planned with local anesthesia and moderate sedation without anesthesia team. One mg of midazolam and 50 µg of fentanyl were given intravenously for conscious sedation. Twenty mL of 2% lidocaine were infiltrated subcutaneously on the left infraclavicular area with an additional 10 mL after the patient complained of local discomfort. Cephalic vein dissection was conducted and contrast venogram via the cephalic vein revealed obstructive axillary-subclavian system, with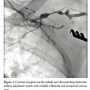 multiple collaterals and unexpected venous stent (Figure 1). The decision was made to switch to the right infraclavicular area. An additional 30 mL of 2% lidocaine was infiltrated and the percutaneous access and lead placement were performed via axillary vein. While closing the device pocket, the patient developed generalized tonic-clonic seizure with stable vital signs. One mg of intravenous midazolam was given. Subsequently, blood pressure, heart rate, and pulse oximetry deteriorated rapidly. She developed another episode of generalized seizure within 5 minutes and her carotid pulse became impalpable. Cardiopulmonary resuscitation was initiated with the diagnosis of pulseless electrical activity. There was no evidence of tension pneumothorax, hemothorax, pericardial effusion, or intravascular injuries. Her initial arterial blood gas revealed mixed metabolic and respiratory acidosis (pH, 7.01; pCO2, 64; pO2, 211; HCO3, 11). The patient regained her pulse after 15 minutes of resuscitation. She recovered rapidly, with complete weaning of vasopressor therapy, and was extubated within 8 hours after the initial event. Lidocaine level drawn during the resuscitation was 8.7 µg/mL (normal, 1.5-5.0 µg/mL). Neurological investigations were negative. The patient was discharged from the hospital 2 days postoperatively with no cardiac or neurological sequalae.
multiple collaterals and unexpected venous stent (Figure 1). The decision was made to switch to the right infraclavicular area. An additional 30 mL of 2% lidocaine was infiltrated and the percutaneous access and lead placement were performed via axillary vein. While closing the device pocket, the patient developed generalized tonic-clonic seizure with stable vital signs. One mg of intravenous midazolam was given. Subsequently, blood pressure, heart rate, and pulse oximetry deteriorated rapidly. She developed another episode of generalized seizure within 5 minutes and her carotid pulse became impalpable. Cardiopulmonary resuscitation was initiated with the diagnosis of pulseless electrical activity. There was no evidence of tension pneumothorax, hemothorax, pericardial effusion, or intravascular injuries. Her initial arterial blood gas revealed mixed metabolic and respiratory acidosis (pH, 7.01; pCO2, 64; pO2, 211; HCO3, 11). The patient regained her pulse after 15 minutes of resuscitation. She recovered rapidly, with complete weaning of vasopressor therapy, and was extubated within 8 hours after the initial event. Lidocaine level drawn during the resuscitation was 8.7 µg/mL (normal, 1.5-5.0 µg/mL). Neurological investigations were negative. The patient was discharged from the hospital 2 days postoperatively with no cardiac or neurological sequalae.
Discussion. Lidocaine is one of the most commonly used infiltration anesthetic agents in cardiovascular procedures including device implantations. Its systemic absorption is determined by the site of injection, dosage and volume, and addition of vasoconstrictor agent. The toxicity is well known to primarily involve the cardiovascular and central nervous system (CNS) and generally does not occur with lidocaine plasma levels less than 10 µg/mL (Table 1). Clinical reports suggest that LAST is very uncommon. Surveys from France and the United States of over 280,000 cases of regional anesthesia report a seizure incidence with epidural injection of approximately 1/10,000 and an incidence of 7/10,000 with peripheral nerve blocks.3,4 The incidence is postulated to be much lower in infiltration anesthesia, since the anesthetic drug level is the lowest when injected into subcutaneous tissue.5
known to primarily involve the cardiovascular and central nervous system (CNS) and generally does not occur with lidocaine plasma levels less than 10 µg/mL (Table 1). Clinical reports suggest that LAST is very uncommon. Surveys from France and the United States of over 280,000 cases of regional anesthesia report a seizure incidence with epidural injection of approximately 1/10,000 and an incidence of 7/10,000 with peripheral nerve blocks.3,4 The incidence is postulated to be much lower in infiltration anesthesia, since the anesthetic drug level is the lowest when injected into subcutaneous tissue.5
Our patient had several risk factors for the development of LAST from lidocaine due to her advanced heart failure (Table 2). Other than those risks, her small body habitus, high vascularized left infraclavicular area facilitating drug absorption, acidosis, and her advanced age were also precipitating factors. The maximum dose of lidocaine is 300 mg or 4.5 mg/kg for plain solution and 500 mg or 7 mg/kg for epinephrine containing lidocaine solution.5 Two percent lidocaine solution is equal to 20 mg/mL. Our patient received a total of 1200 mg, approximately 4 times of her maximum dose. Even though the lidocaine level was 8.7 µg/mL and the CNS and cardiovascular side effects rarely occur at the level less than 10 µg/mL, the patient was in severe mixed metabolic and respiratory acidosis, which favors lidocaine dissociation from the plasma proteins. Increased pCO2 also leads to increased cerebral blood flow and delivery of lidocaine to the brain, and facilitates conversion of the base form of the drugs to the cationic form. The cationic form does not diffuse well across the nerve membrane, so ion trapping will occur, which will increase the apparent CNS toxicity of lidocaine.5
maximum dose. Even though the lidocaine level was 8.7 µg/mL and the CNS and cardiovascular side effects rarely occur at the level less than 10 µg/mL, the patient was in severe mixed metabolic and respiratory acidosis, which favors lidocaine dissociation from the plasma proteins. Increased pCO2 also leads to increased cerebral blood flow and delivery of lidocaine to the brain, and facilitates conversion of the base form of the drugs to the cationic form. The cationic form does not diffuse well across the nerve membrane, so ion trapping will occur, which will increase the apparent CNS toxicity of lidocaine.5
Although the mechanisms is not fully elucidated, it is suspected that when toxicity is expressed as a ventricular arrhythmia, the underlying mechanism likely relates to local anesthetic binding to cardiac sodium channels.6 When cardiac LAST becomes expressed as ventricular contractile depression, other molecular targets are likely to be involved. In cardiomyopathy patients with very poor left ventricular function, further negative inotropic effect may cause significant hemodynamic instability, particularly when it occurs along with metabolic acidosis and hypercapnia.
The best method for avoiding LAST from lidocaine is through prevention. Lidocaine is effective for infiltration in concentrations as dilute as 0.3%-0.5%. Using lidocaine containing epinephrine solution decreases the rate of vascular absorption, thereby allowing more anesthetic molecules to reach the nerve membrane and thus improving the depth and duration of anesthesia.5,7 When toxicity occurs, signs of CNS toxicity will typically occur prior to cardiovascular events. Benzodiazepines are the drug of choice for seizure control. Seizures can increase body metabolism and cause hypoxemia, hypercarbia, and acidosis. It is essential to provide prompt assisted ventilation and circulatory support as needed, to prevent or correct hypercapnia and acidosis and to prevent or correct hypoxemia, which also exacerbates CNS toxicity.8 A novel and effective treatment for severe CNS and cardiac toxicity is the administration of intravenous lipid to theoretically remove the drug from sites of action.6
Conclusion. This case illustrates the importance of preoperatively identifying the risk factors for lidocaine toxicity in advanced heart failure patients undergoing cardiac interventions. Advanced age, cardiac cachexia, advanced left ventricular dysfunction, congestive liver disease, malnutrition, possible central sleep apnea causing respiratory acidosis, and beta-blocker use are common in this population. Preventive measures, such as the use of diluted lidocaine solution of 0.5% with combination of epinephrine, may reduce the risk of LAST. When LAST occurs, benzodiazepine, lipid emulsion therapy, and adequate ventilation and circulatory support are recommended. This case emphasizes the importance of dose adjustment of local anesthetic agents in individual patients, especially in advanced heart failure patients who undergo cardiovascular procedures.
References
- Manolis AS, Maounis T, Vassilikos V, Chiladakis J, Cokkinos DV. Electrophysiologist-implanted transvenous cardioverter defibrillators using local versus general anesthesia. Pacing Clin Electrophysiol. 2000;23(1):96-105.
- Fox DJ, Davidson NC, Royle M, et al. Safety and acceptability of implantation of internal cardioverter-defibrillators under local anesthetic and conscious sedation. Pacing Clin Electrophysiol. 2007;30(8):992-997.
- Brown DL, Ransom DM, Hall JA, Leicht CH, Schroeder DR, Offord KP. Regional anesthesia and local anesthetic-induced systemic toxicity: seizure frequency and accompanying cardiovascular changes. Anesth Analg. 1995;81(2):321-328.
- Auroy Y, Benhamou D, Bargues L, et al. Major complications of regional anesthesia in France: the SOS Regional Anesthesia Hotline Service. Anesthesiology. 2002;97(5):1274-1280.
- Miller RD. Miller's Anesthesia. 7th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2009:3084, I-89.
- Wolfe JW, Butterworth JF. Local anesthetic systemic toxicity: update on mechanisms and treatment. Curr Opin Anaesthesiol. 2011;24(5):561-566.
- Barash PG. Clinical Anesthesia. 6th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2009:1640.
- Neal JM, Bernards CM, Butterworth JFt, Di Gregorio G, Drasner K, Hejtmanek MR, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35(2):152-161.
From the 1Cardiovascular Division, Department of Medicine, University of Miami Miller School of Medicine, Miami, Florida and the 2Department of Family Medicine, University of Iowa Hospitals and Clinics, Iowa City, Iowa.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted July 5, 2013, final version accepted on July 19, 2013.
Address for correspondence: Juan F. Viles-Gonzalez, MD, Assistant Professor, Cardiac Arrhythmia Service, 1295 NW 14th Street, Suite 4062, Miami, FL 33136. Email: j.vilesgonzalez@med.miami.edu


















