Improvement in Pulmonary Vascular Resistance After Relief of Fontan Circuit Obstruction
Abstract: Objectives. Patients with single-ventricle congenital heart disease undergo staged surgical palliations leading to a final Fontan procedure. After Fontan, cardiac index (CI) is primarily determined by pulmonary vascular resistance (PVR). Lower Fontan pressure has been achieved after relieving obstruction within the Fontan circuit, but to date the effect on PVR has not been quantified. We hypothesized that there would be significant reduction in PVR after relief of obstruction within the Fontan circuit; the purpose of this study is therefore to describe the change in PVR after relief of Fontan obstruction. Methods. Retrospective, single-center review of post-Fontan patients who underwent cardiac catheterization with hemodynamics, pulmonary vasodilator testing, and stenting of Fontan circuit obstructions from October 2016 to August 2019. Baseline hemodynamics were obtained on 21% fraction of inspired oxygen (FiO2), followed by administration of 80 ppm inspired nitric oxide (iNO) with repeat hemodynamics. After stenting of Fontan obstructions, hemodynamics were repeated on 21% FiO2. Patient demographics, hemodynamics, CI, and PVR were compared. Results. Twelve patients underwent stenting of Fontan circuit obstructions. There was complete relief of gradient and angiographic obstruction after stent placement in all patients. There was larger decrease in PVR after stent placement compared with iNO administration (32.1% vs 19.3%, respectively; P=.03). Conclusions. This case series provides novel data quantifying the decrease in PVR after relief of Fontan circuit obstruction, suggesting a mechanism for symptomatic improvement after intervention. These data are a compelling addition to the long-term management of this complex patient population.
Key words: congenital heart defects, hemodynamics, stent
Standard therapy for patients with single-ventricle congenital heart disease is to undergo staged surgical palliations leading to a final Fontan procedure.1-3 Unlike patients with a normally functioning biventricular heart, patients with Fontan physiology do not have an unlimited preload reserve to use when in need of increased cardiac output, such as during exercise. As a result of their unique physiology following the Fontan procedure, cardiac output and systemic ventricular preload are entirely reliant on passive pulmonary arterial flow, and even mild increases of pulmonary vascular resistance (PVR) can significantly decrease output in these patients.2-4
Compared with a double-ventricle non-Fontan system, inotropic drugs lack efficacy to increase cardiac index (CI) in patients with Fontan physiology.5,6 However, pulmonary vasodilators, such as sildenafil, appear to improve CI by lowering PVR and can improve clinical outcomes by mitigating conditions that contribute to long-term morbidity and mortality of these patients, including exercise intolerance, protein-losing enteropathy, and plastic bronchitis.7-11
Moreover, recent studies have shown greater improvement in these clinical outcomes after relief of Fontan conduit obstruction compared with pulmonary vasodilators.12 Placing stents within the Fontan circuit has been shown to be safe and effective, and lower pressure in the Fontan circuit has been achieved after successful stent placement.13 To date, however, the effect of relieving obstruction in the Fontan circuit on PVR has not been quantified. We hypothesized that there would be significant reduction in PVR after relief of obstruction within the Fontan circuit; the purpose of the current study is to describe these changes.
Methods
After approval from the University of Arizona institutional review board, who waived the need for informed consent, we performed a retrospective, single-center review of patients with Fontan physiology who underwent cardiac catheterization between October 2016 and August 2019. Inclusion criteria included those with complete hemodynamics, pulmonary vasodilator testing, and stenting of obstruction within the Fontan circuit (conduit and/or branch pulmonary arteries).
All procedures were performed under general anesthesia. Baseline hemodynamics were obtained on 21% fraction of inspired oxygen (FiO2), followed by administration of 80 ppm inspired nitric oxide (iNO) for 5 minutes to test for pulmonary vasoreactivity with repeat hemodynamics.14 Obstruction in the Fontan circuit was defined as angiographic narrowing of the Fontan conduit and/or branch pulmonary arteries (Figure 1A) or by documented pressure gradient. Given the low-flow state within the Fontan circuit, a pressure gradient was not mandatory to define obstruction.15 After stent placement (Figure 1B), hemodynamics were repeated on 21% FiO2. CI was calculated using oxygen consumption from a recently published predictive equation that considers both general anesthesia and single-ventricle physiology.16 As none of the patients had intracardiac shunts, this was also used for pulmonary flow in the calculation of PVR.
Statistical analysis. Data collected included demographics, cardiac diagnoses, and hemodynamics in each condition. Percent increase in CI and percent decrease in PVR on iNO and post stent were also calculated. Comparisons were made with Wilcoxon Signed-Rank test and Friedman’s two-way analysis of variance by ranks, with Bonferroni correction for multiple tests. Statistical analyses were performed using SPSS, version 26 (IBM Corporation).
Results
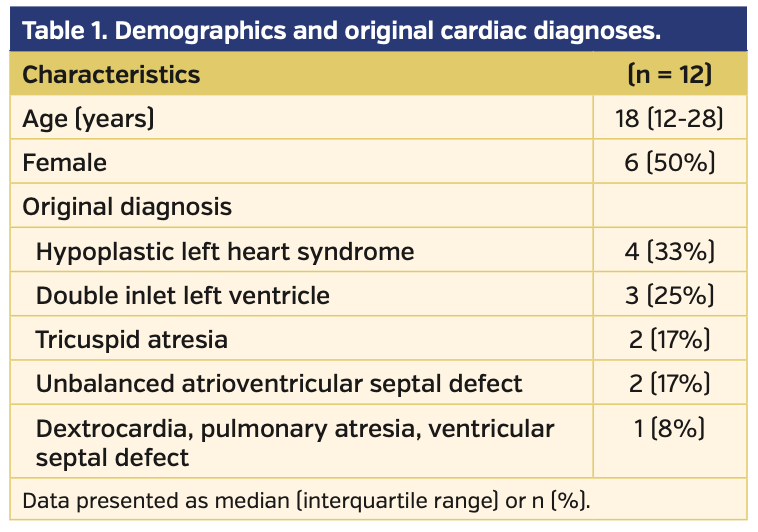
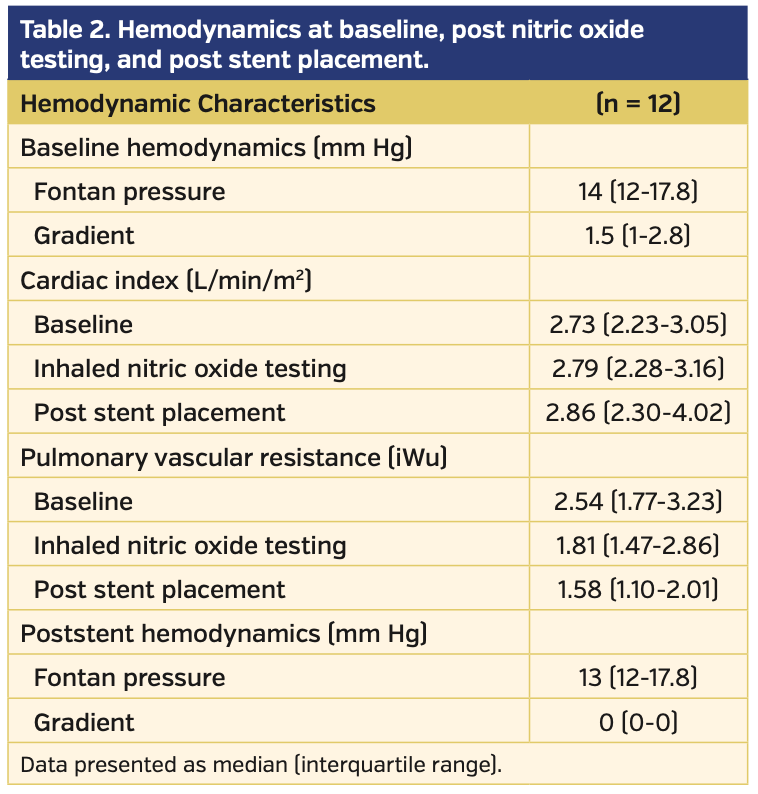
Fourteen patients were identified, of which 12 had complete datasets. Demographics and original cardiac diagnoses are shown in Table 1. Hemodynamics at baseline, at iNO testing, and post stenting are shown in Table 2. All 12 patients underwent percutaneous placement of at least 1 stent within the Fontan circuit — 6 (50%) in the extracardiac Fontan conduit only, 4 (33%) in the left pulmonary artery only, and 2 (17%) in both the Fontan conduit and left pulmonary artery. There were no stent embolisms, vessel injuries, or bleeding complications.
There was complete resolution of the gradient after stent placement in all patients, with no acute change in Fontan pressure (P<.01 and P=.29, respectively) (Table 2). There was a larger decrease in PVR after stent placement compared with iNO administration (32.1% vs 19.3%, respectively; P=.03). In addition, there was a trend toward a larger increase in CI after stent placement compared with iNO administration (17.7% vs 1.8%, respectively; P=.10).
Discussion
In this case series of patients with single-ventricle congenital heart disease with obstruction in the Fontan circuit, we quantified the significant decreases in PVR that occur after relief of the obstruction. While multiple prior studies and the current adult congenital heart disease guidelines support the practice of stenting obstruction in the Fontan circuit, this appears to be the first study quantifying the reduction in PVR that occurs in this setting.
Fontan physiology is markedly different from biventricular circulation. Because there is no subpulmonary ventricle, all flow to the pulmonary vasculature is passive and depends on a careful balance between the pressures of the central venous system and the pulmonary vasculature. One result of total passive venous return is a chronic reduction in preload, which impacts the function of the systemic ventricle, and ultimately contributes to diastolic dysfunction and elevated ventricular filling pressures. This in turn causes impedance of pulmonary blood flow, elevated pulmonary artery pressures, low cardiac output, and systemic venous hypertension. The lack of pulsatile pulmonary arterial flow, pulmonary venous impedance, and nitric oxide dysfunction also contribute to elevated PVR in these patients.3,17
Understanding the mechanisms of dysfunction in this system of passive venous return is important, because PVR plays a major role in the outcome of a patient with Fontan physiology.3 Systemic venous congestion is a primary factor in the pathogenesis of the most challenging complications in Fontan patients, including cirrhosis, protein-losing enteropathy, and plastic bronchitis. Other complications, such as heart failure, dysrhythmia, and death, are also strongly linked to increased PVR and systemic venous pressures.18
Addressing the dysfunction of this system at all points along the pathway has been a focus of congenital cardiologists and cardiothoracic surgeons. One important target of therapy has been to decrease PVR, which plays a large role in the elevation of systemic venous pressures and the pathogenesis of Fontan complications. In recent years, therapies targeting PVR — specifically, pulmonary vasodilator medications — have shown promising improvements for patients with Fontan physiology, including increased exercise tolerance, increased myocardial performance, and in some cases, symptomatic improvement of plastic bronchitis and protein-losing enteropathy.19 It is unknown, however, what the effects of long-term use of pulmonary vasodilators would be, and alternative methods of decreasing PVR could prove critical in the management, and potentially the prevention, of Fontan complications. Our study quantifies the benefits of one alternative to long-term pulmonary vasodilator use.
The incidence of obstruction within the Fontan circuit has been reported to be as high as 75% with polyethylene terephthalate extracardiac conduits.20 An obstructed Fontan conduit directly contributes to the impendence of venous flow, the consequences of which can significantly impact the survival of these patients.21 In order to mitigate these complications, another surgical or percutaneous intervention may be required to relieve the obstruction. Previous studies have evaluated the outcome of stenting within the Fontan circuit, demonstrating significant symptomatic improvement in factors such as exercise tolerance, cyanosis, and peripheral edema.22 Our data provide further insight into the physiologic improvements that occur after percutaneous stent placement, specifically, the impact on PVR.
The reduction in PVR documented in the current study provides a mechanism to explain the improvements in clinical symptomatology. In Fontan physiology, improvements in PVR lead to improved CI and likely decreased systemic venous hypertension, allowing for improved drainage of lymph fluid with less edema and ascites and potentially less lymphatic leak in the setting of plastic bronchitis or protein-losing enteropathy.2,3 This will allow for improved decision making when considering surgical procedures in the high-risk population with Fontan physiology to decrease rates of morbidity and mortality.
Study limitations. There are several limitations to the current study. The retrospective nature may not allow for rigorous control of all potential confounders. In addition, the relatively small sample size likely led to the lack of statistically significant differences in CI. Pre- and postcatheterization cardiopulmonary exercise testing was not available for all patients to assess clinical improvement, but this has been reported previously. Despite the small number of patients, the documented changes in PVR are quite compelling and can provide the stimulus for prospective studies on the topic.
Conclusion
We have provided novel data quantifying the decrease in PVR after relief of Fontan circuit obstruction, suggesting a mechanism to explain previous reports of increased exercise tolerance and improvement in plastic bronchitis and protein-losing enteropathy. Larger studies will likely confirm the significance of these findings, but these data are a compelling addition to the long-term management of this complex patient population.
From 1the University of Arizona College of Medicine-Tucson, Tucson, Arizona; 2the University of Arizona Department of Pediatrics, Tucson, Arizona; and 3the University of Arizona Department of Pediatrics (Cardiology), Tucson, Arizona.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
The authors report that patient consent was provided for publication of the images used herein.
Manuscript accepted March 31, 2020.
Address for correspondence: Michael D. Seckeler, MD, MSc, University of Arizona, Department of Pediatrics (Cardiology), P.O. Box 245073, 1501 N. Campbell Ave, Tucson, AZ 85724. Email: mseckeler@peds.arizona.edu
- Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240-248.
- Gwelling M, Brown S. The Fontan circulation after 45 years: update in physiology. Heart. 2016;102:1081-1086.
- Gwelling M, Brown S, Eyskens B, et al. The Fontan circulation: who controls cardiac output? Interact Cardiovasc Thorac Surg. 2010;10:428-433.
- Dasi LP, Krishnankuttyrema R, Kitajima HD, et al. Fontan hemodynamics: importance of pulmonary artery diameter. J Thorac Cardiovasc Surg. 2009;137:560-564.
- Sorensen GK, Ramamoorthy C, Lynn AM, French J, Stevenson JG. Hemodynamic effects of amrinone in children after Fontan surgery. Anesth Analg. 1996;82:241-246.
- Williams DB, Kiernan PD, Schaff HV, Marsh HM, Danielson GK. The hemodynamic response to dopamine and nitroprusside following right atrium-pulmonary artery bypass (Fontan procedure). Ann Thorac Surg. 1982;34:51-57.
- Giradini A, Balducci A, Specchia S, Gargiulo G, Bonvicini M, Picchio FM. Effect of sildenafil on hemodynamic response to exercise and exercise capacity in Fontan patients. Eur Heart J. 2008;29:1681-1687.
- Ovaert C, Thijs D, Dewolf D, et al. The effect of bosentan in patients with a failing Fontan circulation. Cardiol Young. 2009;11:1-9.
- Apostolopoulou SC, Papagiannis J, Rammos S. Bosentan induces clinical, exercise and hemodynamic improvement in a pre-transplant patient with plastic bronchitis after Fontan operation. J Heart Lung Transplant. 2005;24:1174-1176.
- Uzun O, Wong JK, Bhole V, Stumper O. Resolution of protein-losing enteropathy and normalization of mesenteric Doppler flow with sildenafil after Fontan. Ann Thorac Surg. 2006;82:e39-e40.
- Khambadkone S, Li J, de Leval MR, Cullen S, Deanfield JE, Redington AN. Basal pulmonary vascular resistance and nitric oxide responsiveness late after Fontan-type operation. Circulation. 2003;107:3204-3208.
- Undik ten Cate FEA, Trieschmann U, Germund I, et al, Stenting the Fontan pathway in paediatric patients with obstructed extracardiac conduits. Heart. 2017;103:1111-1116.
- Noonan P, Kudumula V, Anderson B, et al. Stenting of the left pulmonary artery after palliation of hypoplastic left heart syndrome. Catheter Cardiovasc Interv. 2016;88:225-232.
- Milger K, Felix JF, Voswinckel R, et al. Sildenafil versus nitric oxide for acute vasodilator testing in pulmonary arterial hypertension. Pulm Circ. 2015;5:305-312.
- Stout K, Daniels C, Aboulhosn J, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease. J Am Coll Cardiol. 2019;73:e81-e192.
- Seckeler MD, Hirsch R, Beekman RH 3rd, Goldstein BH. A new predictive equation for oxygen consumption in children and adults with congenital and acquired heart disease. Heart. 2015;101:517-524.
- Egbe AC, Connolly HM, Taggart NW, Al-Otaibi M, Borlaug BA. Invasive and noninvasive hemodynamic assessment in adults with Fontan palliation. Int J Cardiol. 2018;254:96-100.
- Egbe AC, Reddy YNV, Khan AR, et al. Venous congestion and pulmonary vascular function in Fontan circulation: implications for prognosis and treatment. Int J Cardiol. 2017;271:312-316.
- Snarr BS, Paridon SM, Rychik J, Goldberg DJ. Pulmonary vasodilator therapy in the failing Fontan circulation: rationale and efficacy. Cardiol Young. 2015;25:1489-1492.
- van Brakel TJ, Schoof PH, de Roo F, Nikkels PG, Evens FC, Haas F. High incidence of Dacron conduit stenosis for extracardiac Fontan procedure. J Thorac Cardiovasc Surg. 2014;147:1568-1572.
- Rychik J, Atz AM, Celermajer DS, et al; American Heart Association Council on Cardiovascular Disease in the Young and Council on Cardiovascular and Stroke Nursing. Evaluation and management of the child and adult with Fontan circulation: a scientific statement from the American Heart Association. Circulation. 2019;140:234-284.
- Lee SY, Song MK, Kim GB, et al. Relation between exercise capacity and extracardiac conduit size in patients with Fontan circulation. Pediatr Cardiol. 2019;40:1584-1590.