Impella 2.5 Assisted Balloon Aortic Valvuloplasty and Percutaneous Coronary Intervention as a Bridge to Heart Transplantation
Abstract: Percutaneous left ventricular assist device (pLVAD) utilization is increasing as the potential applications expand. We report a case of high-risk balloon aortic valvuloplasty and percutaneous coronary intervention using the Impella 2.5 pLVAD in a patient with severely depressed left ventricular function as a bridge to heart transplantation.
J INVASIVE CARDIOL 2012;24(5):229-230
Key words: percutaneous left ventricular assist device, aortic stenosis, left main dissection
_______________________________________________
In the era of transcatheter aortic valve implantation (TAVI) and left ventricular device destination therapy, balloon aortic valvuloplasty (BAV) as bridge therapy is resurgent. Concomitantly, percutaneous left ventricular assist device (pLVAD) utilization is increasing. Use of pLVAD as an adjunct to BAV has been limited to date.
Case Report
 A 62-year-old man was admitted for acute decompensated congestive heart failure with New York Heart Association (NYHA) class IV symptoms requiring milrinone inotropic support and diuresis. After 5 days of therapy, he was compensated and no longer oxygen dependent, but remained inotrope dependent with persisting NYHA class IV symptoms. He had a prior history of ischemic cardiomyopathy with left ventricular ejection fraction of 13%, prior 3-vessel coronary artery bypass grafting in 1999 (left internal mammary artery
A 62-year-old man was admitted for acute decompensated congestive heart failure with New York Heart Association (NYHA) class IV symptoms requiring milrinone inotropic support and diuresis. After 5 days of therapy, he was compensated and no longer oxygen dependent, but remained inotrope dependent with persisting NYHA class IV symptoms. He had a prior history of ischemic cardiomyopathy with left ventricular ejection fraction of 13%, prior 3-vessel coronary artery bypass grafting in 1999 (left internal mammary artery 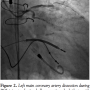 to obtuse marginal 1, right internal mammary artery to left anterior descending artery, saphenous vein graft to right coronary) and critical symptomatic aortic stenosis (AS) (aortic valve area 0.43 cm2 and mean gradient 40 mm Hg) twice treated with aortic valvuloplasty over the last 2 years. The patient had recently been considered and then declined for TAVI as part of the US Partner trial. On preprocedure exam, the patient was thin, with a body mass index (BMI) of 23, an old, well-healed sternotomy scar, a heart rate of 70 bpm and a blood pressure of 90/60 mm Hg on milrinone. There was a prominent harsh III/VI ejection systolic murmur of aortic stenosis at the second intercostal space radiating throughout the precordium to the apex. Lungs were clear to auscultation and extremities were well perfused, with no edema and 1+ pulses in the lower extremities. Our heart transplant service referred him for coronary angiography with possible percutaneous coronary intervention (PCI) and BAV as a bridge to left ventricular assist device or cardiac transplantation.
to obtuse marginal 1, right internal mammary artery to left anterior descending artery, saphenous vein graft to right coronary) and critical symptomatic aortic stenosis (AS) (aortic valve area 0.43 cm2 and mean gradient 40 mm Hg) twice treated with aortic valvuloplasty over the last 2 years. The patient had recently been considered and then declined for TAVI as part of the US Partner trial. On preprocedure exam, the patient was thin, with a body mass index (BMI) of 23, an old, well-healed sternotomy scar, a heart rate of 70 bpm and a blood pressure of 90/60 mm Hg on milrinone. There was a prominent harsh III/VI ejection systolic murmur of aortic stenosis at the second intercostal space radiating throughout the precordium to the apex. Lungs were clear to auscultation and extremities were well perfused, with no edema and 1+ pulses in the lower extremities. Our heart transplant service referred him for coronary angiography with possible percutaneous coronary intervention (PCI) and BAV as a bridge to left ventricular assist device or cardiac transplantation.
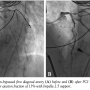
 Cardiac catheterization from the right common femoral artery demonstrated that all 3 grafts were patent. A diseased non-bypassed diagonal artery was identified as a PCI target (Figure 1A) with the goal of improving left ventricular function. After angiography demonstrated adequate femoral artery dimensions, access to the left common femoral artery was obtained and the aortic valve was crossed using the standard technique with an Amplatz left (AL2) catheter. Baseline aortic valve gradient was 43 mm Hg. An Impella 2.5 (Abiomed) was
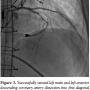
Cardiac catheterization from the right common femoral artery demonstrated that all 3 grafts were patent. A diseased non-bypassed diagonal artery was identified as a PCI target (Figure 1A) with the goal of improving left ventricular function. After angiography demonstrated adequate femoral artery dimensions, access to the left common femoral artery was obtained and the aortic valve was crossed using the standard technique with an Amplatz left (AL2) catheter. Baseline aortic valve gradient was 43 mm Hg. An Impella 2.5 (Abiomed) was  placed. Baseline cardiac output and capillary wedge pressure were 4.5 L/min and 25 mm Hg, respectively. Two minutes after placement of the Impella 2.5, these parameters improved to 6.0 L/min and 18 mm Hg, respectively and systemic blood pressure as measured in the proximal aorta improved to 110-120/55-70 mm Hg. The 90% proximal stenosis of the first diagonal artery was then successfully treated by PCI with a 2.5 x 28 mm drug-eluting stent (Figure 1B). Subsequently, a spiral left main (LM) guide dissection was identified (Figure 2). The patient remained hemodynamically stable. The protected left anterior descending (LAD) artery and unprotected diagonal were wired and PCI from the diagonal artery into the proximal LAD and the LM with 2.5 x 28 mm and 3.5 x 15 mm stents was successfully undertaken (Figure 3). The aortic valve was then crossed with a second wire and successful BAV with a 20 x 6 mm Z-Med II Balloon (NuMED, Inc) was performed (Figure 4). Rapid pacing of the right ventricle was not necessary. The patient remained hemodynamically stable throughout the whole procedure. The Impella was left in for 24 hours before being weaned and removed. The patient was discharged on home inotropes. At 6-week follow-up exam in the heart transplant clinic, he remained stable with NYHA III symptoms and was listed for heart transplant as a United Network of Organ Sharing (UNOS) Status IB. At 8 weeks, he underwent successful heart transplant surgery.
placed. Baseline cardiac output and capillary wedge pressure were 4.5 L/min and 25 mm Hg, respectively. Two minutes after placement of the Impella 2.5, these parameters improved to 6.0 L/min and 18 mm Hg, respectively and systemic blood pressure as measured in the proximal aorta improved to 110-120/55-70 mm Hg. The 90% proximal stenosis of the first diagonal artery was then successfully treated by PCI with a 2.5 x 28 mm drug-eluting stent (Figure 1B). Subsequently, a spiral left main (LM) guide dissection was identified (Figure 2). The patient remained hemodynamically stable. The protected left anterior descending (LAD) artery and unprotected diagonal were wired and PCI from the diagonal artery into the proximal LAD and the LM with 2.5 x 28 mm and 3.5 x 15 mm stents was successfully undertaken (Figure 3). The aortic valve was then crossed with a second wire and successful BAV with a 20 x 6 mm Z-Med II Balloon (NuMED, Inc) was performed (Figure 4). Rapid pacing of the right ventricle was not necessary. The patient remained hemodynamically stable throughout the whole procedure. The Impella was left in for 24 hours before being weaned and removed. The patient was discharged on home inotropes. At 6-week follow-up exam in the heart transplant clinic, he remained stable with NYHA III symptoms and was listed for heart transplant as a United Network of Organ Sharing (UNOS) Status IB. At 8 weeks, he underwent successful heart transplant surgery.
Discussion
Use of pLVAD in patients with AS has been limited to date. A few series report successful use of the TandemHeart pLVAD in the setting of AS patients with cardiogenic shock1 and AS patients undergoing BAV.2,3 However, AS has been an exclusion in clinical trials studying the Impella pLVAD due to concerns of the potential impact of the 12 Fr cannula across the aortic valve. The successful use of an Impella pLVAD as an adjunct to BAV has only recently been reported4 and it was on the basis of this experience that the above case was undertaken. In our case, there did not appear to be any adverse events resulting from the use of the Impella for BAV, even after the potentially life-threatening complication of a LM guide dissection. Hemodynamic compromise could be minimized by undertaking the BAV prior to the PCI. However, a similar argument can also be made for staging the PCI first, since BAV-related complications might be less likely after coronary revascularization; with the added benefit that if the BAV is deferred due to unforeseen PCI complications, the patient is not committed to large-bore arterial access. Prior reports tend to favor coronary angioplasty followed by BAV.5 We surmised that right ventricular (RV) pacing would not be necessary due to the juxtaposition of the Impella catheter to the valvuloplasty balloon at the valvular orifice providing mechanical support against movement and/or as a consequence of the hemodynamic impact of the unloading of the left ventricle. Though true in our case, it was not in the only other previously reported case.4
This case raises a number of interesting topics that can only be addressed with further study: (1) What are the in vivo hemodynamics with a 12 Fr Impella cannula astride a diseased aortic valve orifice and what are the possible risks and benefits? (2) Having proven viable, in what circumstances should Impella-assisted BAV be undertaken and should it be favored over other pLVADs?
References
- Gregoric ID, Loyalka P, Radovancevic R, et al. TandemHeart as a rescue therapy for patients with critical aortic valve stenosis. Ann Thorac Surg 2009;88(6):1822-1826.
- Tanaka K, Rangarajan K, Azarbal B, Tobis JM. Percutaneous ventricular assist during aortic valvuloplasty: potential application to the deployment of aortic stent-valves. Tex Heart Inst J. 2007;34(1):36-40.
- Thomas JL, Al-Ameri H, Economides C, et al. Use of a percutaneous left ventricular assist device for high-risk cardiac interventions and cardiogenic shock. J Invasive Cardiol. 2010;22(8):360-364.
- Londono JC, Martinez CA, Singh V, O’Neill WW. Hemodynamic support with Impella 2.5 during balloon aortic valvuloplasty in a high-risk patient. J Interv Cardiol. 2011;24(2):193-197.
- McKay RG, Safian RD, Berman AD, et al. Combined percutaneous aortic valvuloplasty and transluminal coronary angioplasty in adult patients with calcific aortic stenosis and coronary artery disease. Circulation. 1987;76(6):1298-1306.
_______________________________________________
From the Department of Cardiovascular Diseases, The John Ochsner Heart & Vascular Institute, Ochsner Clinic Foundation, New Orleans, Louisiana.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Badawi is a grant recipient from Abiomed’s “Most Challenging Case Workshop.” Dr Grise is a consultant for Abiomed. No other authors report conflicts of interest regarding the content herein.
Manuscript submitted November 30, 2011, provisional acceptance given January 17, 2012, final version accepted January 24, 2012.
Address for correspondence: Dr Ramy Badawi, Queen’s Heart Physician Practice, The Queen’s Medical Center, 550 S. Beretania Street, Suite 601, Honolulu, HI 96813. Email: rbadawi@msn.com
















