High Incidence of Inaccurate Stent Placement in the Treatment of Coronary Aorto-Ostial Disease
ABSTRACT: Objectives. The purpose of this study was to evaluate the incidence of inaccurate stent positioning in the treatment of coronary aorto-ostial lesions. Background. The percutaneous treatment of aorto-ostial disease is challenging, with a paucity of data describing the incidence of stent mispositioning. Methods. We retrospectively reviewed the accuracy of stent positioning in 100 consecutive coronary aorto-ostial lesions. Using careful angiographic review, each stent placement was classified as “missed” (> 1 mm distal or proximal to the angiographically determined ostium) or “accurately” positioned. Results. The true ostium was missed during stent placement in 54% of cases. In 52% of the misses, the stent was placed too proximally. This proximal miss was associated with an inability to coaxially re-engage the treated vessel in 93% of the cases. The stent was placed too distally in 48% of missed cases, resulting in a placement of one or more additional overlapping stents in 38% of those cases. Clinical follow-up (mean, 24.5 ± 12.9 months) was obtained in 98% of cases. Angiographic follow-up prompted by recurrent chest pain or ischemia was performed in 45/100 cases. There was a three-fold increase in restenosis and target lesion revascularization (TLR) among the cohort of patients with stent misplacement (26% and 23%, respectively) compared to those with accurate stent placement (9% and 6%, respectively; p = 0.02 for both restenosis and TLR). Conclusions. Angiographically-guided stenting for coronary aorto-ostial disease leads to a high incidence of proximal and distal stent misplacement. Stent mispositioning is associated with significantly higher restenosis and clinically driven TLR compared to patients with accurate stent placement.
J INVASIVE CARDIOL 2011;23:322–326
Key words: stenting, aorto-ostial disease, stent placement, percutaneous coronary intervention
__________________________________________
The percutaneous interventional treatment of aorto-ostial disease is challenging. The therapeutic approach to the treatment of aorto-ostial disease has evolved over the years, from balloon angioplasty to debulking therapies with excimer laser or rotational atherectomy to stenting, and in some cases a combination of several of these modalities.
There is a paucity of information reporting the technical success and long-term outcomes following stent-based intervention to treat coronary aorto-ostial lesions. Prior studies have reported low technical success rates, possibly related to stent misplacement, as well as relatively high complication rates, increased restenosis rates, and reduced event-free survival.1–4
The technical approach to stenting these lesions using angiographic guidance is often time-consuming and frustrating. The reliance upon angiographic landmarks may be associated with frequent and recognizable stent misplacement.5 In response to these difficulties, one report has described the use of an additional guidewire, placed outside the guiding catheter tip, to assist with accurate stent positioning. However, this “Szabo” technique has not been widely adopted, and has been used with modest success.6,7
The optimal strategy for treating aorto-ostial stenoses has yet to be determined. The initial reports describing the use of a nitinol-based stent-positioning tool (the “Ostial Pro”, Ostial Solutions, Kalamazoo, Michigan) appear promising, but the long-term outcomes have not yet been reported.5,8 To date, there have been relatively few data published to evaluate the accuracy of stent placement to treat aorto-ostial disease. Some preliminary data on this subject have suggested a high incidence of stent misplacement.5 The primary objective of the current study was to better ascertain the incidence of stent misplacement in this challenging lesion subset.
Methods
We retrospectively reviewed the angiograms and clinical outcomes in 100 consecutive patients treated with stent implantation of native coronary artery or bypass graft aorto-ostial (> 50%) lesions. This case series includes all coronary aorto-ostial lesions treated with stenting, as identified by our database, between June 2005 and July 2007. Nine experienced interventional cardiologists performed the interventions in this registry (average experience, 20.7 years; average career coronary percutaneous coronary intervention [PCI] volume, 3,650 ± 456 cases; average annual case volume, 174 ± 38 cases/year).
All treated aorto-ostial lesions were > 50% diameter stenosis (visual, angiographic assessment) and involved the ostium of a coronary artery, a mammary artery bypass graft, or a venous bypass graft. Ostial lesions of the left anterior descending and left circumflex arteries were not included unless they had a separate ostium arising directly from the aorta. The target vessel had to be large enough to accommodate a stent (reference diameter ≥ 2.25 mm). Patients with ST-elevation myocardial infarction were excluded. No patients were excluded due to other anatomical considerations (e.g., vessel calcification, anomalous origin, etc.).
In all cases, coronary angiography demonstrated the target aorto-ostial lesion in at least one projection with minimal foreshortening. Direct stenting was the preferred method of stent deployment, and was utilized in 63% of the cases. A majority of the cases underwent post-dilatation in an effort to “flare” or “trumpet” the stent struts against the aortic wall and maximize luminal diameter (86%).
An anatomic “miss” or stent “misplacement” was defined as the stent being deployed > 1 mm proximal or distal to the true ostium. The data collected included: 1) the vessel treated; 2) classification as accurate, versus distal or proximal stent misplacement; 3) the ability to re-engage the treated vessel (if too proximal); 4) additional stent(s) placement, when distal misplacement was recognized and treated with a second stent; 5) post-procedural TIMI flow; 6) major acute complications (including death, myocardial infarction, perforation, and stent embolization); 7) angiographic restenosis in those patients who had follow-up angiography for any reason; and 8) target lesion revascularization (TLR) during follow up.
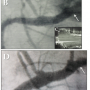
 Stent implantation was categorized as: 1) too distal (> 1 mm distal to the angiographically determined ostium with > 30% residual stenosis) or requiring a second proximal stent (Figure 1); 2) too proximal (> 1 mm proximal to the angiographically determined ostium, particularly if the ostium could not be re-engaged with a guiding or diagnostic catheter; or 3) accurate (< 1 mm from the true ostium, with the ability to re-engage the ostium and with good reflux of the contrast on angiography) (Figure 2).
Stent implantation was categorized as: 1) too distal (> 1 mm distal to the angiographically determined ostium with > 30% residual stenosis) or requiring a second proximal stent (Figure 1); 2) too proximal (> 1 mm proximal to the angiographically determined ostium, particularly if the ostium could not be re-engaged with a guiding or diagnostic catheter; or 3) accurate (< 1 mm from the true ostium, with the ability to re-engage the ostium and with good reflux of the contrast on angiography) (Figure 2).
Three experienced interventional cardiologists reviewed the operative reports and angiograms independently, while blinded to any long-term follow-up data. A consensus, or a majority of two of the three reviewers, had to be in agreement with regard to the primary study endpoint regarding “accurate” or “missed” stent positioning. The distal misses were angiographically obvious using the high-resolution digital angiography available for all cases. Although proximal misses may be less certain angiographically, the inability to completely and coaxially engage or re-engage the target vessel with the guiding catheter or a diagnostic catheter was one of the key indicators of a proximal miss and was observed in 93% of the cases so categorized. In 91% of cases, there was consensus among the three independent and blinded film reviewers with regard to the categorization as missed versus accurate. There was 100% agreement in missed cases regarding whether it was a proximal or distal miss.
 Data related to fluoroscopy time, procedure time, and the amount of contrast utilized, which included contrast used during the diagnostic angiography, were collected in all cases.
Data related to fluoroscopy time, procedure time, and the amount of contrast utilized, which included contrast used during the diagnostic angiography, were collected in all cases.
Forty-five of the patients underwent angiographic restudy for recurrent symptoms from restenosis, or for other indications, such as atypical chest pain or a positive noninvasive test. Longitudinal clinical follow-up was obtained in 98/100 patients (98%).
Statistics. An unpaired t-test was used to compare the procedural data and the follow-up outcomes of restenosis and TLR between the optimal and missed stent placement groups. A p-value of ≤ 0.05 was considered statistically significant.
Results
The distribution of aorto-ostial lesions treated included the right coronary artery (n = 60), left main coronary artery (n = 10), saphenous vein graft (n = 26), left internal mammary artery graft (n = 3), and an anomalous left anterior descending coronary artery arising from a separate ostium (n = 1). A single stent was placed in a majority (90%) of the aorto-ostial lesions. The aorto-ostial stents placed included 80 drug-eluting stents (DES) and 20 bare-metal stents (BMS). There was no difference in the percentage or ratio of DES versus BMS in the stent misplacement group (44 DES and 10 BMS) compared to the accurate placement group (36 DES and 10 BMS) (p = NS). There was no difference in the incidence of diabetes mellitus in the missed versus the accurately-placed stent groups (30% versus 32%, respectively).
Clinical follow up (mean, 24.5 ± 12 .9 months) was obtained in 98% of cases. Using the definition from this registry, the stent placement missed the true ostium in 54/100 cases (54%). The vessel distribution and incidence of these misses were as follows: right coronary artery (32/60; 53%), left main coronary artery (3/10; 30%), saphenous vein graft (17/26; 65%), left internal mammary artery (1/3; 33%), and left anterior descending artery (1/1; 100%).
Forty-eight percent of the patients in the missed subgroup and 41% of the accurately placed stent group underwent a single-vessel intervention, with no other intervention other than the ostial stent placed during the index PCI. In 28/54 missed cases (52%), the stent was placed too proximally, such that the stent was placed clearly projecting outside the coronary ostium and into the aorta. As a consequence, there was an inability to adequately and coaxially re-engage the treated vessel in 26/28 (93%) of these proximal miss cases. Furthermore, opacification of the target vessel with contrast injection was suboptimal in the majority of postprocedural angiograms among the patients in this subset.
The stent was deployed too distally in 48% of the missed cases. This was recognized by the operator in most cases, resulting in further intervention with at least one additional stent placed proximal to the first stent in 10/26 of these cases (38%). Interestingly, the second stent was placed too proximally in 5/10 of the restented cases (50%), resulting in the inability to reengage the ostium of treated vessel in all 5 of these “double miss” cases. Additional balloon angioplasty to resolve residual ostial stensosis was performed in an additional 55% of cases. For the purpose of calculating the stent placement “miss rate,” the double miss cases were only counted as a single miss.
The ostial stent position was judged as accurate in 46/100 cases (46%). The incidence of accurate stent positioning was 47% in the right coronary artery (28/60), 70% in the left main artery (7/10), 35% in the saphenous vein grafts (9/26), and 66% in the left internal mammary artery (2/3).
Postprocedural TIMI III flow was observed in 98% of all treated vessels. In two of the saphenous vein graft stent cases, there was TIMI II flow at the conclusion of the procedure. Both of these cases were large vein grafts (i.e., > 4 mm in diameter). Embolic protection was utilized in 1 of 2 cases. One of these 2 cases had a CPK-MB elevation, meeting the criteria for a non-Q wave myocardial infarction. There were no other periprocedural major adverse cardiac events. The only other procedural complication was an asymptomatic, localized, type A dissection in the proximal right coronary artery in a case with a distal stent miss.
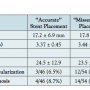 The average stent diameters and lengths are shown in Table 1, and were, respectively, 3.44 ± 0.65 mm and 17.8 ± 7.8 mm in the missed group versus 3.37 ± 0.45 mm and 17.2 ± 6.9 mm in the accurate group (p = NS). There were no significant differences in stent length, stent diameter, or the duration of follow-up among the patients with stent misplacement versus accurate stent placement.
The average stent diameters and lengths are shown in Table 1, and were, respectively, 3.44 ± 0.65 mm and 17.8 ± 7.8 mm in the missed group versus 3.37 ± 0.45 mm and 17.2 ± 6.9 mm in the accurate group (p = NS). There were no significant differences in stent length, stent diameter, or the duration of follow-up among the patients with stent misplacement versus accurate stent placement.
When comparing fluoroscopy time, procedure time, and contrast use, we only analyzed the data for the single-vessel interventions due to the difficulty in controlling for these variables if additional lesions were treated. The average fluoroscopy time for all of these cases was 21.6 ± 8.8 minutes. The fluoroscopy time was 21.0 ± 9.2 minutes for misses versus 22.1 ± 7.4 minutes in cases with accurate stent placement (p = NS).
The average procedure time for the single-vessel interventions was 59.2 ± 16.9 minutes (55.8 ± 18.3 minutes for misses versus 62.6 ± 20.1 minutes for optimally placed stents [p = NS]). The average amount of contrast utilized was 229 ± 48 ml (223 ± 46 ml for misses versus 234 ± 57 ml for accurate stent placement [p = NS]).
Follow-up angiography for recurrent symptoms and/or documented ischemia was performed in 45/100 cases (45%) (n = 19 for accurate versus n = 26 for misses). Unstable or progressive angina was the presenting diagnosis in a majority (76%) of the angiographic follow-up cases. Non-ST elevation myocardial infarction was observed at presentation in 9/45 (20%) of the angiographic follow-up cases.
There was a significant relationship between stent misplacement and the adverse results of restenosis (26% in the miss group versus 9% in the accurate group) and TLR (22.5% versus 6.5%). This represents a three-fold increase in both of these outcomes in patients with stent misses compared to those patients with more accurately placed aorto-ostial stents (p = 0.02 for higher restenosis and higher TLR in the missed group).
Seven of 12 TLRs in the missed group were from distal misses and 5/12 were observed in the proximal miss group (TLR was 5/28 in the proximal miss group [18%], versus 7/26 [27%] in the distal miss group [p = NS]). Thus, there was a trend to suggest that distal misses are more prone to restenosis, but the series did not have the statistical power to prove this.
Of the 26 patients with initial stent misplacement who had angiographic follow up, 14/26 (53%) had restenosis versus 4/19 (21%) with restenosis among the accurate placement group who had angiographic follow up (p = 0.011). Repeat revascularization (TLR) was performed in 12/26 (46%) of these initially missed cases compared to 3/19 (16%) of the accurate group (p = 0.011).
The revascularization success rate in these TLR cases was 11/12 (92%). There was an inability to adequately re-engage the ostial right coronary artery in a proximal miss case, leading to TLR failure in 1 case. In 3 of the 4 other proximal miss cases associated with TLR, there were difficulties in re-engagement of the proximal missed stent. In 1 case, rotational atherectomy had to be used to drill though proximal stent struts in order to achieve a successful TLR. Successful repeat stenting was performed in all 7 distal miss TLR cases.
There were no documented cases of early or late stent thrombosis in this cohort. There were no statistically significant differences in death or myocardial infarction among the two cohorts.
The remainder of the patients (n = 55) who did not have angiographic follow up were followed clinically and did not have recurrent symptoms warranting angiography at a mean follow up of 24.5 ± 12.9 months.
Discussion
Aorto-ostial disease represents approximately 7% of all coronary stent interventions and more than 90% of renal artery interventions.9,10 Although aorto-ostial lesions represent a relatively small minority of treated coronary lesions, the overall prevalence of stenting for aorto-ostial disease is not rare. It is estimated that there are approximately 300,000 aorto-ostial stent procedures performed worldwide each year.5,9,10
The immediate- and long-term results with percutaneous intervention for aorto-ostial lesions have generally been poor, despite the use of a variety of interventional techniques and tools.2–20 Although stent placement with BMS and more recently DES may have improved outcomes, the recurrent stenosis rate remains relatively high.
The precise placement of stents in the aorto-ostial position poses a technical challenge to the interventional cardiologist, and may account for suboptimal long-term outcomes with stenting.1–5,8 The current study describes our single center’s experience with the implantation of coronary stents in the treatment of aorto-ostial lesions. This is the largest consecutive case series ever reported to address the issue of the accuracy of stent placement for these lesions. The primary finding of this study was that there is a very high (> 50%) incidence of stent misplacement when using angiographic guidance, even among experienced interventional cardiologists.
Stents are felt to be a good choice for percutaneous treatment of aorto-ostial disease due to their ability to limit both acute elastic recoil and late negative remodeling.9 When compared to percutaneous transluminal coronary angioplasty (PTCA) or atherectomy, stents have been found to improve short- and long-term outcomes in the treatment of aorto-ostial disease. Nonetheless, the risk of restenosis of these lesions still remains high.11 The findings of the current study suggest that at least one reason for the relatively high restenosis rate seen with both BMS, and more recently DES, may be related to stent misplacement.
It is generally acknowledged that aorto-ostial stents are difficult to place in an optimal position. Conventional angiographic guidance may be misleading in many cases. The guiding catheter is often slightly engaged into the target vessel, even though the operator believes that the guide is aligned at the true ostium. This results in a distal miss in many cases.
Some operators intentionally stick the proximal stent edge well into the aorta in order to prevent a distal miss. This, of course, almost always will create a proximal miss. Unfortunately, as demonstrated in our study, a proximal miss will result in a poor outcome for the patient, related to an inability to coaxially re-engage the ostium of a major coronary artery or graft again with either a diagnostic or guiding catheter in almost all cases. This may pose a greater risk than the restenosis that is often observed after a distal miss (Figure 1).
The anatomic challenges in placing aorto-ostial stents may also be associated with greater fluoroscopy time and with greater contrast use. At our institution, the average fluoroscopy time for aorto-ostial stenting was 21.6 ± 8.8 minutes versus 9.3 ± 3.7 minutes for single-stent, non-ostial lesions (n = 50) (p < 0.005). The average amount of contrast utilized for a single-vessel aorto-ostial intervention was 229 ± 48 ml, compared to 167 ± 31 ml for a non-aorto-ostial intervention (p < 0.005). Thus, fluoroscopy times were more than 60% greater and the amount of contrast used was approximately 75% greater in aorto-ostial lesions compared to single-lesion (non-ostial) stenting.
Missing the ostium during stent implantation may have serious adverse consequences. If the stent is placed too distal to the lesion, there appears to be a high incidence of restenosis. If a second stent is required, the risk of restenosis as well as stent thrombosis may be increased. In addition, there is the added cost and time involved in placing an additional overlapping stent.4 If the stent is placed proximally, re-engagement of a diagnostic or guiding catheter may be suboptimal or impossible. This may make it difficult or nearly impossible to perform future angiography or percutaneous intervention if necessary.4
The suboptimal results observed in the treatment of aorto-ostial lesions is particularly important when one considers the extensive area of myocardium typically supplied by the vessel distal to an ostial lesion. This is particularly true in stenting of unprotected left main lesions. The association of the aorto-ostial lesion with myocardial infarction and sudden cardiac death was demonstrated by the elaborate work of Rissanen.9 In this necropsy series, significant ostial stenosis (> 50%) was identified in 53% of patients with a recent myocardial infarction. In addition, ostial stenosis was identified in 42% of 89 cases of sudden death without a recent myocardial infarction.9
The restenosis rates following PCI in coronary ostial lesions have been reported to be significantly higher than in non-ostial lesions.3 In a study by Rocha-Singh et al, the overall restenosis rate was 32% after stenting of aorto-ostial stenoses in native coronaries and saphenous vein grafts, despite a 93% procedural success rate. In that study, the restenosis rate was 35% for aorto-ostial saphenous vein graft lesions.3 Our observation of a lower TLR in cases with accurate stent positioning suggests that correct stent positioning at the ostium may decrease elastic recoil and create a larger final luminal diameter, which may be associated with an improved restenosis rate.13,18
Study limitations. Although this study represents the largest consecutive series describing stent positioning in coronary aorto-ostial lesions, it is a retrospective, observational study conducted at a single center. The possibility exists that risk factors not identified in this study may have added prognostic information. Very high-quality digital angiography was used with a team of three interventional physicians reviewing each film to determine the accuracy of stent placement. Despite this, there are some limitations regarding the accuracy of angiographic rather than IVUS assessment. The high rate of failed coaxial engagement of the target vessel after proximal misses does provide strong evidence of stent protrusion in those cases identified as proximal misses. Distal misses were quite evident angiographically and often were recognized and treated with one or more additional stents or repeated balloon dilatation. We believe that those assessments are very accurate using our team review methodology.
Clinical and angiographic follow-up data were not available for all patients. Angiographic repeat studies were clinically driven, and therefore were not obtained in all 100 cases. There was a statistically significant impact of stent misplacement predicting higher restenosis and TLR rates, but not of sufficient power to determine if this would translate into differences in cardiac death or myocardial infarction. Finally, this was not a randomized trial assigning patients to accurate versus inaccurate placement. Performing such a randomized trial would be unrealistic and unethical.
Conclusion
The treatment of aorto-ostial stenotic lesions is technically challenging. The major contributors to the challenge are technical and anatomical. The limitations of angiographically guided stent placement are clearly demonstrated in this study. Inaccurate stent positioning was observed in more that half of the cases. This study is unique among all previous studies examining aorto-ostial lesions, by defining both the incidence of stent misplacement and demonstrating the adverse clinical outcome of a significantly higher rate of TLR associated with stent misplacement. These findings suggest that there is a need for tools or techniques to improve the accuracy of positioning of stents to treat aorto-ostial disease.
References
- Mathias DW, Mooney JF, Lange HW, et al. Frequency of success and complications of coronary angioplasty of a stenosis at the ostium of a branch vessel. Am J Cardiol 1991;67:491–495.
- Horlitz M, Amin FR, Sigwart U, Clague JR. Coronary stenting of aorto-ostial saphenous vein graft lesions. J Invasive Cardiol 2006;68:901–906.
- Rocha-Singh K, Morris N, Wong SC, et al. Coronary stenting for treatment of ostial stenoses of native coronary arteries or aortocoronary saphenous venous grafts. Am J Cardiol 1995;75:26–29.
- Tierstein P, Stratienko AA, Schatz RA. Coronary stenting for ostial stenosis: Initial results and six-month follow-up. Circulation 1991;84(Suppl 2):II-250.
- Fischell TA, Saltiel FS, Foster MT, et al. Initial clinical experience using an ostial stent positioning system (Ostial Pro™) for the accurate placement of stents in the treatment of coronary aorto-ostial lesions. J Invasive Cardiol 2009;21:53–59.
- Szabo S, Abramowitz B, Vaitkus PT. New technique for aorto-ostial stent placement. Am J Cardiol 2005;96:212H.
- Kern MJ, Ouellette D, Frianeza T. A new technique to anchor stents for exact placement in ostial stenosis: The stent tail wire or Szabo technique. Catheter Cardiovasc Interv 2006;68:901–906.
- Fischell TA, Malhotra S, Khan S, Bonan R. A new ostial stent positioning system (Ostial Pro™) for the accurate placement of stents to treat aorto-ostial lesions. Catheter Cardiovasc Interv 2008;71:353–357.
- Rissanen V. Occurrence of coronary ostial stenosis in a necropsy series of myocardial infarction, sudden death, and violent death. Brit Heart J 1975;37:182–191.
- Darabian S, Amirzadegan AR, Sadeghian H, et al. Ostial lesions of left main and right coronary arteries: Demographic and angiographic features. Angiology 2008;59:682–687.
- Kaplan S, Barlis P, Tanigawa J, et al. Unconventional treatment of aorto-ostial in-stent restenosis with marked protrusion into the aorta. J Cardiovasc Med (Hagerstown) 2008;9:184-186.
- Teirstein P, Stratienko AA, Schatz RA. Coronary stenting for ostial stenosis: Initial results and six-month follow-up. Circulation 1991;84(Suppl 2):II-250.
- Zampieri P, Colombo A, Almagor Y, et al. Results of coronary stenting of ostial lesions. Am J Cardiol 1994;73:901–903.
- Colombo A, Adamian M. Cutting balloon angioplasty of ostial coronary lesions: Do we need the stent? J Invasive Cardiol 1999;11:231–232.
- Muramatsu T, Tsukahara R, Ho M, et al. Efficacy of cutting balloon angioplasty for lesions at the ostium of the coronary arteries. J Invasive Cardiol 1999;11:201–206.
- Topol EJ, Ellis SG, Fishman J, et al. Multicenter study of percutaneous transluminal angioplasty for the right coronary ostial stenosis. J Invasive Cardiol 1987;9:1214–1218.
- Kerwin PM, McKeever LS, Marek JC, et al. Directional atherectomy of aorto-ostial stenosis. Cathet Cardiovasc Diagn 1993;1(Suppl 1):17–25.
- Caputo RP, Chafizadeh ER, Stoler RC, et al. Stent jail: A minimum security prison. Am J Cardiol 1996;77:1226–1229.
- Harb TS, Ling FS. Inadvertent stent extraction six months after implantation by an entrapped cutting balloon. Catheter Cardiovasc Interv 2001;53:415–419.
- Corrozza JP, Kuntz RE, Levine MJ, et al. Angiographic and clinical outcome of intracoronary stenting: Immediate and long-term results from a large single-center experience. J Am Coll Cardiol 1992;20:328–332.
__________________________________________
From the 1Borgess Heart Institute and 2Borgess Research Institute, Kalamazoo, Michigan.
Dr. Fischell reports that he is a cofounder and shareholder of Ostial Solutions, LLC.
Submitted May 9, 2011, provisional acceptance given May 27, 2011, final version accepted June 3, 2011.
Address for correspondence: Tim A. Fischell, MD, Professor of Medicine, Michigan State University, Borgess Heart Institute, 1521 Gull Road, Kalamazoo, MI 49048. Email: tafisc@gmail.com
















