Femoral Artery Chronic Total Occlusion Revascularization (FACTOR) Score and Algorithm: Feasibility and Validation in a Single-Center Study of Femoropopliteal Occlusions
Abstract: Objectives. To develop and validate a hybrid algorithm to approach complex superficial femoral artery (SFA) chronic total occlusions (CTOs) in a step-wise fashion. Background. SFA-CTO represents one of the most challenging subsets of lower-extremity peripheral arterial disease. Depending on lesion characteristics, successful percutaneous crossing of the occluded segment may prove to be very difficult. Methods. We retrospectively evaluated all consecutive patients with SFA-CTO at our institution. The included patients had baseline Rutherford category (2-4) symptoms and were graded using the femoral artery chronic total occlusion revascularization (FACTOR) score. Multiple modalities (wire-based strategies, CTO devices, re-entry devices) were used to cross the occlusions based on the proposed FACTOR algorithm. Primary endpoint was technical success, defined as successful CTO crossing. Results. A total of 150 patients (mean age, 71 years) with SFA-CTO were retrospectively reviewed to evaluate the feasibility and utility of the FACTOR score and algorithm in a single center across multiple experienced operators. Following the FACTOR algorithm, overall procedural success was achieved in 143 out of 150 patients (95%). Successful antegrade CTO crossing occurred in 59%; success rates increased to 85% when additional retrograde popliteal, tibiopedal, and direct SFA accesses were used. In multivariate analysis, retrograde wire crossing, stent placement, and atherectomy were independent predictors of successful revascularization. Conclusions. The results of our study show that utilization of the FACTOR score and algorithm can result in high rates of successful SFA-CTO revascularization.
Key words: chronic total occlusion, CTO, drug-coated balloon, drug-eluting balloon, peripheral angiography
Lower-extremity peripheral arterial disease (PAD) has a broad range of clinical presentations ranging from asymptomatic disease to intermittent claudication or critical limb ischemia.1,2 Superficial femoral artery (SFA) and popliteal artery disease account for a substantial amount of PAD seen in clinical practice, with up to 40% of all symptomatic patients having an underlying chronic total occlusion (CTO).3,4 Although advancements in endovascular therapies have allowed for effective percutaneous approach to some of the most complex lesions (TASC C and D), revascularization of CTOs still remains a challenge.5-7
The rate of success in percutaneous treatment of complex SFA-CTOs has been constrained by several procedure-specific factors. One of the main factors is the tendency to spend too much time using one approach (ie, contralateral retrograde) rather than switching to an alternative access point. CTO interventions often require prolonged procedure times and may result in repeat procedures, with potential for multiple admissions.8 Furthermore, there is frequently an increase in the use of contrast and radiation exposure to the patient and interventionalist.9 Finally, attempting complex SFA-CTOs may lead to vascular complications such as arterial dissection, vessel perforation, or compromise of collateral circulation.10
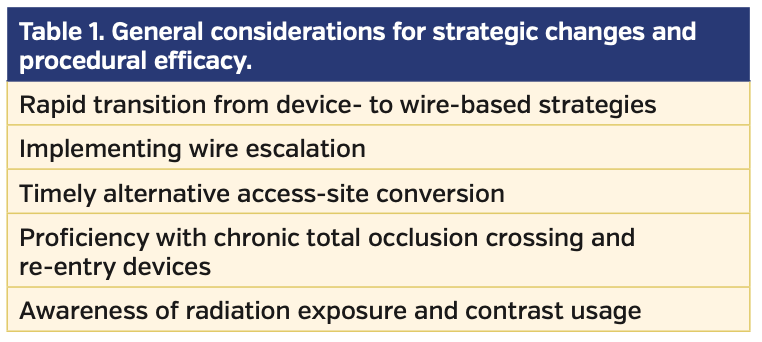
Fundamentals and learning curve. Given the above challenges, some interventional operators may not be comfortable or adequately trained to perform complex endovascular interventions proficiently. Although a large number of vascular training programs have incorporated PAD intervention into their curriculum, confounding variables such as low case volumes, inconsistent operator experience, limited availability of advanced endovascular devices, unfamiliarity with obtaining alternative access sites, and competitive relationships between various specialties may negatively impact the overall competence in performing complex CTO interventions.11 These general, as well as procedural variables, need to be taken into consideration to facilitate a successful CTO program (Table 1).
Previous studies of CTO percutaneous coronary interventions have demonstrated that success rates improve as a function of incremental experience without a concomitant increase in adverse events.12 By extension, physicians can gain experience in peripheral CTO interventions by enrolling in a training program that offers contact with endovascular experts for hands-on training on advanced devices, attend regional and/or national endovascular meetings to remain up to date on the latest technology and treatment strategies, invite proctors for complex SFA-CTO cases, and develop a therapeutic algorithm to swiftly navigate the challenges and available options for complex CTO revascularization.
Indications for SFA-CTO revascularization. Prior to planning a revascularization strategy for an SFA-CTO, anticipation of clear clinical benefit from revascularization must be established. Patients who are likely to benefit from intervention include those with symptoms of lifestyle-limiting claudication (despite the use of optimal medical therapy and a dedicated exercise program) or evidence of critical limb ischemia with rest pain or non-healing ulceration.13
Lesion stratification and development of a predictive SFA-CTO score. In planning a percutaneous revascularization strategy for SFA-CTO, the arterial anatomy must be taken into consideration. Several lesion-specific characteristics are thought to affect the overall likelihood of successful crossing of the occlusion; these include the proximal cap morphology, lesion length, severe vessel calcification, presence of collaterals at proximal and distal CTO cap, and a prior failed attempt at CTO recanalization. These characteristics are analogous to most of the previously described parameters thought to affect the successful crossing of coronary CTOs.14
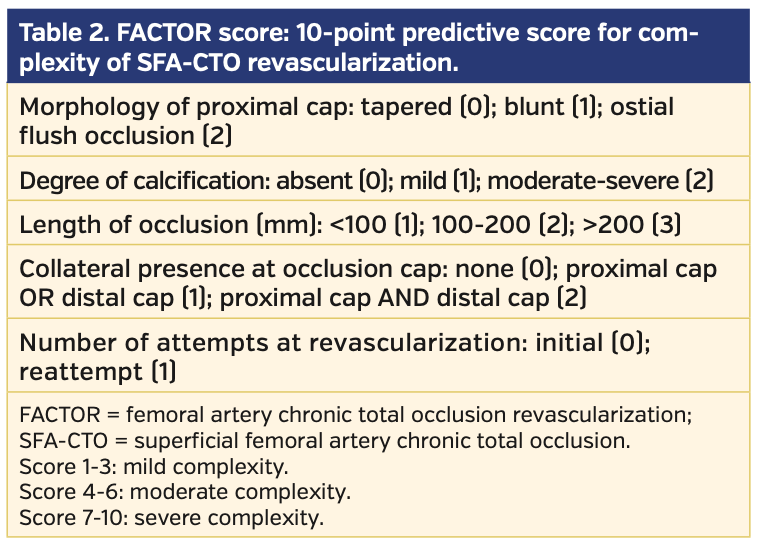
The proximal cap morphology determines how effectively a wire or CTO device may engage the occlusion in an antegrade fashion. A tapered cap is more favorable than a blunt proximal cap, whereas an ostial SFA flush occlusion may obviate an antegrade approach entirely and instead necessitate retrograde popliteal or tibiopedal access. Longer lesion lengths are also less favorable for successful CTO crossing, as they increase the chance of subintimal wire entry and subsequent need for re-entry techniques or devices. Severe vessel calcification also promotes entry into the subintimal space and may hinder antegrade progression of devices, requiring retrograde arterial access. Additionally, the presence of collateral vessels in the proximal or distal cap may impede successful antegrade or retrograde crossing by deflecting the wire or device into the collateral rather than the true CTO. A prior failed attempt to revascularize an SFA-CTO inherently raises lesion difficulty and may prompt more aggressive approaches, such as direct SFA retrograde access or transcollateral crossing. Based on these criteria, a femoral artery chronic total occlusion revascularization (FACTOR) score was established to better assess the complexity and revascularization success rate for SFA-CTOs (Table 2).
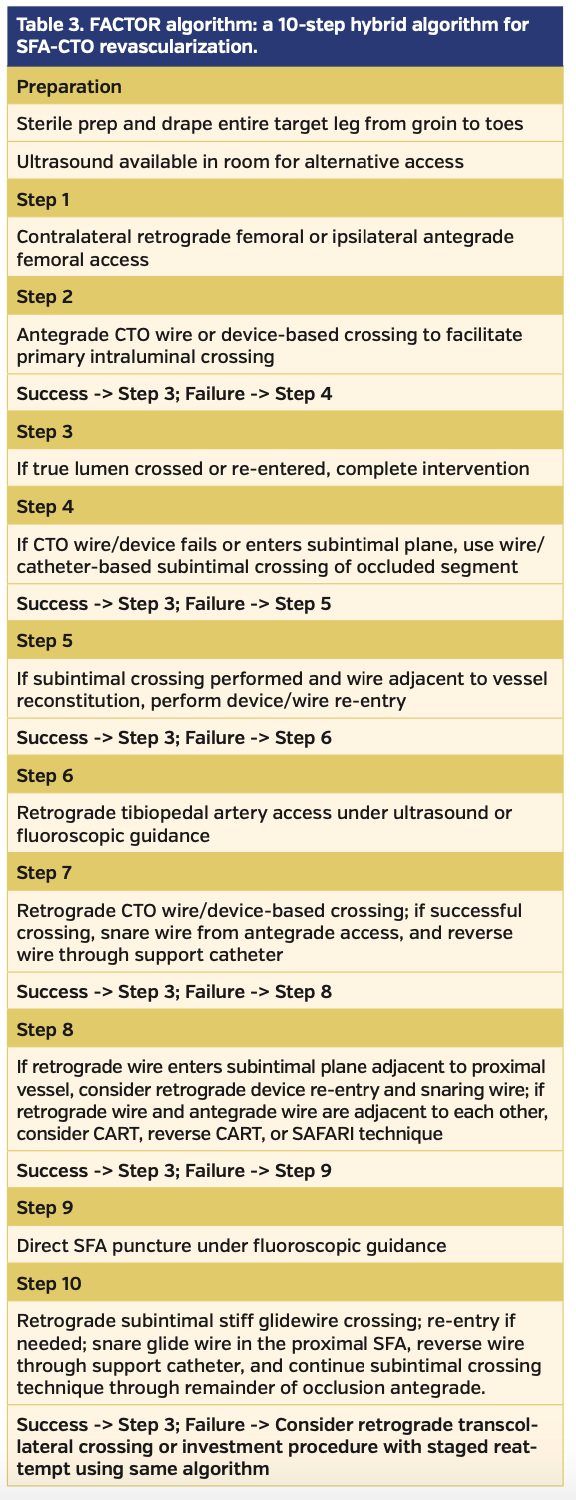
Development of an algorithm for SFA-CTO revascularization. The benefits of having a procedural algorithm to improve success rates and efficiency while minimizing complications have been previously outlined in the coronary CTO literature.14 Some of the main considerations include rapid transition to alternative techniques, proficiency with dedicated CTO wires and devices, and awareness of radiation exposure and contrast levels (Table 1). Recently, Banerjee et al developed a consensus document to describe a “PCTO algorithm” meant to simplify the approach to both femoropopliteal and below-the-knee CTOs.15 We therefore propose a hybrid FACTOR algorithm, which is based on the effective use of alternative-access strategies to increase the success rate of SFA-CTO revascularization (Table 3).
The first goal of the proposed algorithm is to simplify, facilitate, and navigate through the steps and seamlessly progress to complete CTO revascularization (Figure 1). The second goal is to prepare the operator for a standardized hybrid approach with adequate knowledge of alternative access options and the myriad revascularization techniques available if faced with initial failure to cross. Our final goal is to validate the success of the proposed algorithm by evaluating the crossing success rates as a function of the FACTOR score.
Guidewire technology and wire escalation. Successfully crossing an SFA-CTO using wire-based strategies often necessitates wire escalation; therefore, a fundamental knowledge of peripheral guidewires is of paramount importance. First, the core diameter of the guidewire should be considered, with the standard sizes of 0.014˝, 0.018˝, and 0.035˝. The core material (either stainless-steel or nitinol) results in significant differences in guidewire properties, such as rigidity, flexibility, and torqueability. Stainless-steel wires are often more rigid, with better torque transmission, whereas nitinol wires are more flexible and have kink-resistant tips. Hybrid wires are also useful, combining high tensile stainless-steel shafts and nitinol tips. The core taper of a wire addresses the ability to steer, track, and prolapse. Short tapers allow for better support at shorter distances, but at the expense of increased likelihood of prolapse; on the other hand, long gradual tapers track well around bends, but provide less support. A core that extends to the tip of the wire provides better tactile feedback, improved steerability, and increased durability. A core that does not extend to the tip is more flexible, easier to shape, more likely to prolapse, and ideal for navigating tortuous anatomy. Different levels of guidewire tip load (in grams) provide more or less ability to penetrate an occlusion cap.16
Wire-based crossing techniques. When intraluminal antegrade wire crossing initially fails, retrograde access is typically used in an effort to cross the occlusion through the distal cap, which can often be less dense than the proximal cap. Depending on tibiopedal vessel size, a 4 Fr or 5 Fr sheath can be inserted, after which an 0.014˝ or 0.018˝ wire supported by an angled catheter can achieve successful retrograde wire-based crossing.
If retrograde approach also fails, several techniques can be used to facilitate successful crossing. The subintimal arterial flossing with antegrade-retrograde intervention (SAFARI) technique involves using both antegrade and retrograde wires within subintimal planes to create a common channel through which recanalization can be completed.17 If the antegrade and retrograde wires are adjacent to each other within the occlusion, controlled antegrade retrograde subintimal tracking (CART) or reverse CART technique can be utilized. This maneuver involves balloon inflation in either antegrade or retrograde direction within the lesion to facilitate plane disruption and subsequent wire crossing from the opposite direction. This technique has been shown to be safe and effective and has similar long-term outcomes when compared with simple antegrade wire crossing.18
Another reported technique in SFA-CTO intervention is transcollateral crossing.19 Primarily used in below-the-knee occlusions, this technique can be used to perform retrograde wire-based crossing by utilizing vessel-to-vessel arterial collaterals. This technique, however, should be used with extreme caution, as damaging collaterals to the distal vasculature may progress a claudicant limb to an acutely ischemic limb, possibly requiring surgical bailout. A 0.014˝ hydrophilic wire and dedicated tapered-tip, coated, small-vessel wires likely offer the best chance for successful transcollateral crossing.
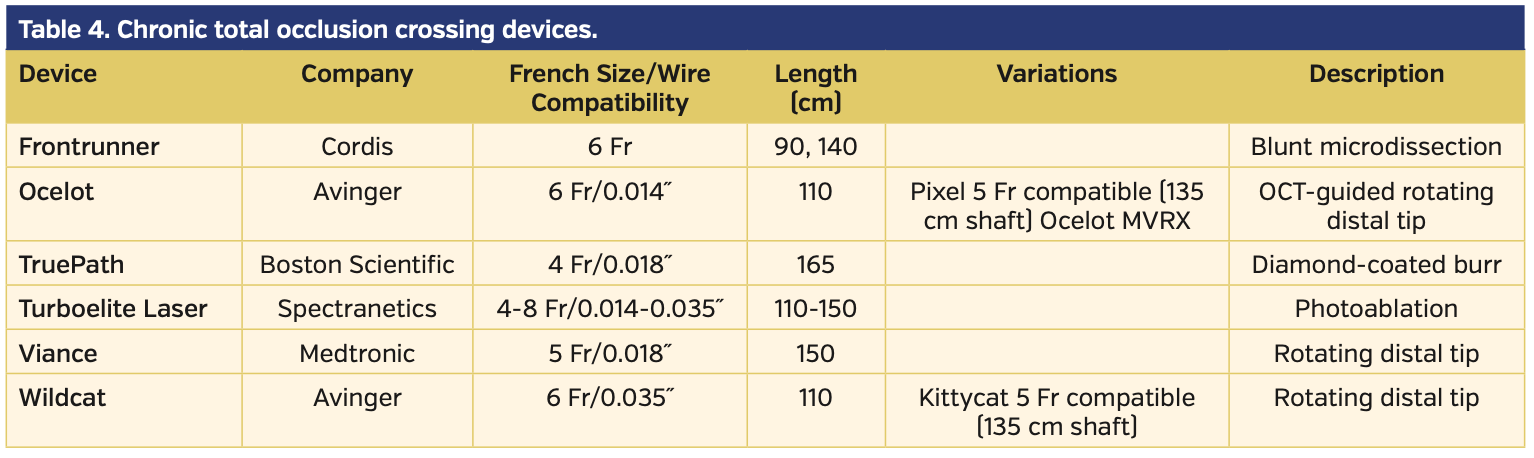
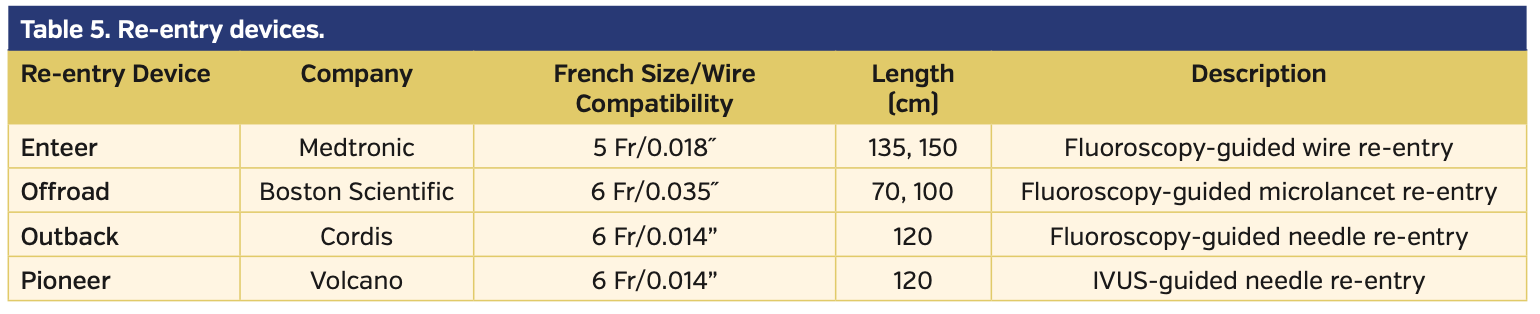
Device-based crossing and re-entry. Although wire-based crossing is most frequently adopted, several CTO devices have been evaluated and approved for use in SFA-CTOs.20-24 Of note, a recent study demonstrated that the use of dedicated CTO crossing devices provides significantly higher technical success and lower reintervention and amputation rates.25 The assortment of currently available CTO crossing devices is enumerated in Table 4. If the CTO device fails to enter the true lumen distally but is positioned within a subintimal plane adjacent to the reconstituted lumen, various re-entry devices have been approved to facilitate re-entry.26-28 These devices are enumerated in Table 5.
Additionally, if adjunctive SAFARI technique has been employed with antegrade and retrograde wires in the subintimal plane adjacent to each other, a balloon can be inflated within the subintimal space from retrograde direction, and a re-entry device used to puncture the balloon from an antegrade direction. The wire is then advanced antegrade into the balloon and externalized, and revascularization is completed according to operator preference.29
Mastering alternative arterial access sites. Alternative access-site proficiency is paramount to increasing procedural success. The FACTOR algorithm calls for sterile drape and preparation of the entire treatment limb, from groin to toes, to allow for rapid transition from an antegrade approach to retrograde access from any site in the target limb. Ultrasound availability in the procedure room is also imperative for retrograde access, whether it be popliteal, tibiopedal, or direct SFA. Although angiography can be used to facilitate retrograde arterial access, ultrasound guidance is the preferred strategy given the increase in radiation exposure inherent to complex endovascular procedures.
In the past, popliteal artery access required placing the patient in a prone position during the procedure, leading to increased procedural time, patient discomfort, and possible contamination of previously placed arterial access sites. Instead of placing the patient in a prone position, “frog leg” positioning of the treatment limb can allow the patient to remain supine while still allowing successful ultrasound-guided popliteal artery access.30-32 The advantage of popliteal artery access over tibiopedal access is the fact that the popliteal artery can allow the insertion of larger sheaths and use of a wider variety of CTO crossing options.
Failed antegrade attempts of recanalization can be approached retrogradely from tibiopedal access with low acute and late complication rates.33,34 Ultrasound-guided access of the posterior tibial and dorsalis pedis arteries can be successfully performed with the patient remaining in a supine position. Typically, a 4 Fr or 5 Fr sheath is inserted; however, 4/5 Fr or 5/6 Fr Slender Glidesheaths (Terumo) can also be used correspondingly without resulting in a larger arteriotomy. An 0.014˝ or 0.018˝ wire/catheter system, as well as CTO devices, can be used for retrograde crossing, depending on anatomy and sheath size. Once successful CTO crossing is achieved with either an 0.014˝ or 0.018˝ wire supported by a corresponding microcatheter, the intervention can be completed retrogradely if a large-enough retrograde sheath is used,35 otherwise the retrograde wire would have to be snared, externalized, and reversed for antegrade revascularization.36
The tibiopedal arterial minimally invasive (TAMI) technique has been previously described as an option to perform the entire peripheral intervention using only a tibiopedal access.37 Insertion of a 6 Fr sheath is optimal and would accommodate most CTO crossing and atherectomy devices. Diagnostic angiography must be performed using a catheter with its distal tip positioned proximal to the level of disease. Alternatively, radial artery access can be obtained for diagnostic imaging, while vessel treatment is performed from the tibiopedal access.38 The TAMI technique may serve as a useful option for patients unable to tolerate common femoral artery (CFA) access due to body habitus or comorbidities, with the added advantage of decreased postprocedural bleeding risk. Following sheath removal, a radial artery hemostasis device can be used to obtain successful hemostasis over the pedal artery access site.36
If both antegrade and retrograde attempts at CTO crossing fail, consideration may be given to direct SFA access as a third alternative access option.39,40 The goal is to access the SFA retrogradely (under fluoroscopic or ultrasound guidance) in an area of the CTO that has not been reached by wires or devices from prior access sites. Vessel calcification or prior stent placement is favorable, as this would aid in determining whether or not a direct SFA retrograde wire is following the general SFA architecture on fluoroscopy. Once access is achieved, a stiff Glidewire with a support catheter is advanced in a retrograde fashion to cross the occlusion; this is often performed without sheath insertion. Although no contrast injections can be performed, the wire/support catheter are advanced to the proximal SFA or proximal cap for re-entry, as described previously. After re-entry, snaring, and externalization, a support catheter taken over the externalized wire is used to reverse the approach to antegrade. The Glidewire can then be withdrawn from the direct SFA access site and a wire is advanced through the support catheter past the access site to cross the remainder of the SFA occlusion, employing previously described wire- and device-based techniques.
Direct SFA access has a particular advantage during attempts at revascularization of occluded SFA stents.39 The presence of the stent precludes subintimal crossing or use of re-entry techniques without compromising the integrity of the previously placed stents. Wires or devices may preferentially go around the stent edge, instead of through the occluded stent. Fluoroscopic guidance is used to guide a needle into the SFA stent, and a stiff Glidewire introduced for retrograde crossing, as described earlier.
The final alternative access site into an occluded segment of the SFA is via robust collateral pathways, described as a transcollateral approach.19 This technique has been further elucidated earlier in the wire-based crossing techniques section.
Methods
We retrospectively evaluated all consecutive patients with SFA-CTO that underwent percutaneous revascularization at our institution. All patients were reviewed to evaluate the feasibility and utility of the FACTOR score and algorithm in a single center across multiple experienced operators. The FACTOR score was calculated based on the presence of the anatomical factors detailed above. The included patients had baseline Rutherford category 2-4 limb ischemia and were assigned a respective FACTOR score (Table 2). Multiple modalities (wire-based strategies, CTO devices, re-entry devices) were used to cross the occlusions and multiple access points were also employed when necessary for procedural success based on the proposed FACTOR algorithm (Table 3). The primary endpoint of the study was technical success, defined as successful crossing of the SFA-CTO segment. Secondary endpoints included evaluation of predictors of successful CTO crossing.
Statistical analysis. We conducted a step-wise logistical regression analysis of successful CTO revascularization. The variables included in the model were male sex, history of coronary artery disease, dyslipidemia, diabetes mellitus, tobacco abuse, CTO length, FACTOR score, retrograde wire crossing, direct SFA access, retrograde access approach, antegrade access approach, contralateral femoral approach, stent placement, atherectomy, cutting-balloon angioplasty, and number of access sites. All categorical variables were compared using Chi-square test. Continuous variables were reported as mean ± standard deviation and categorical variables were reported as frequencies. Statistical significance was defined as P<.05.
Results
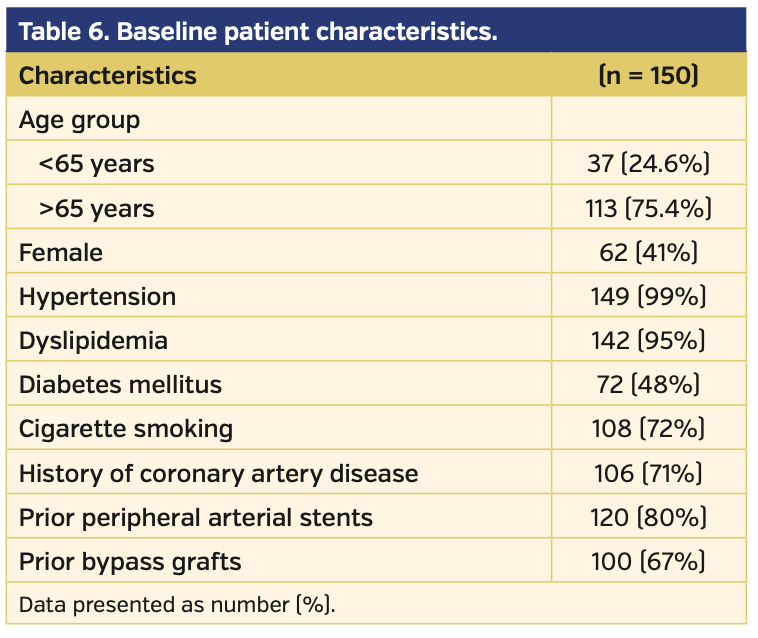
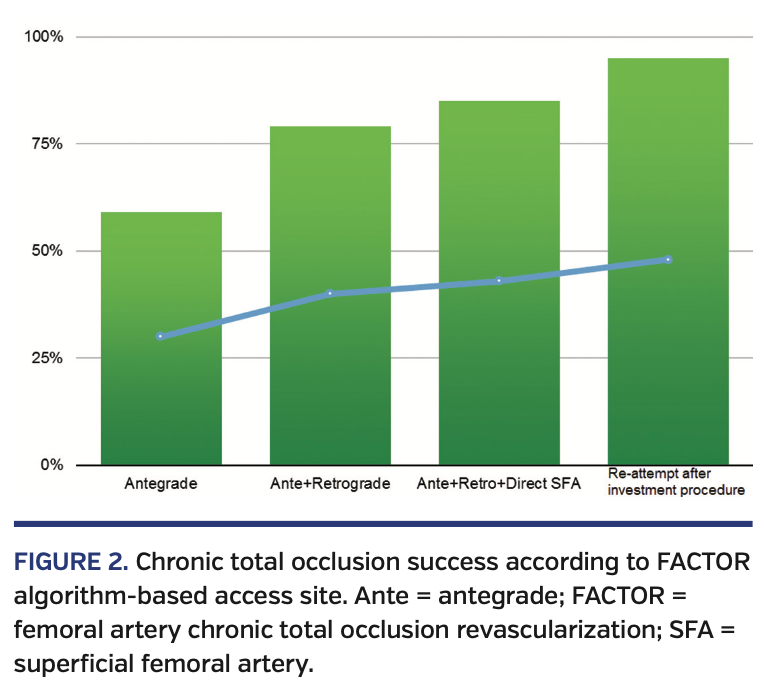
A total of 150 patients who underwent endovascular intervention of SFA-CTO lesions were included in our study. Baseline characteristics of the patient population are listed in Table 6. The average FACTOR score of the included patients was 5.2, suggesting a moderately complex cohort of lesions. Following application of the FACTOR algorithm, the primary endpoint of overall procedural success was achieved in 143 patients (95%) (Figure 1). Successful antegrade CTO crossing occurred in 59% of patients. When antegrade crossing failed and retrograde access (popliteal or tibiopedal access) was used, the crossing success rate increased to 79%. If both antegrade and retrograde approaches failed, use of direct SFA access as a third access point increased the crossing success rate to 85%. Fifteen procedures failed to cross the CTO regardless of access site; following a repeat attempt at intervention, the overall success rate increased to 95% (Figure 2).
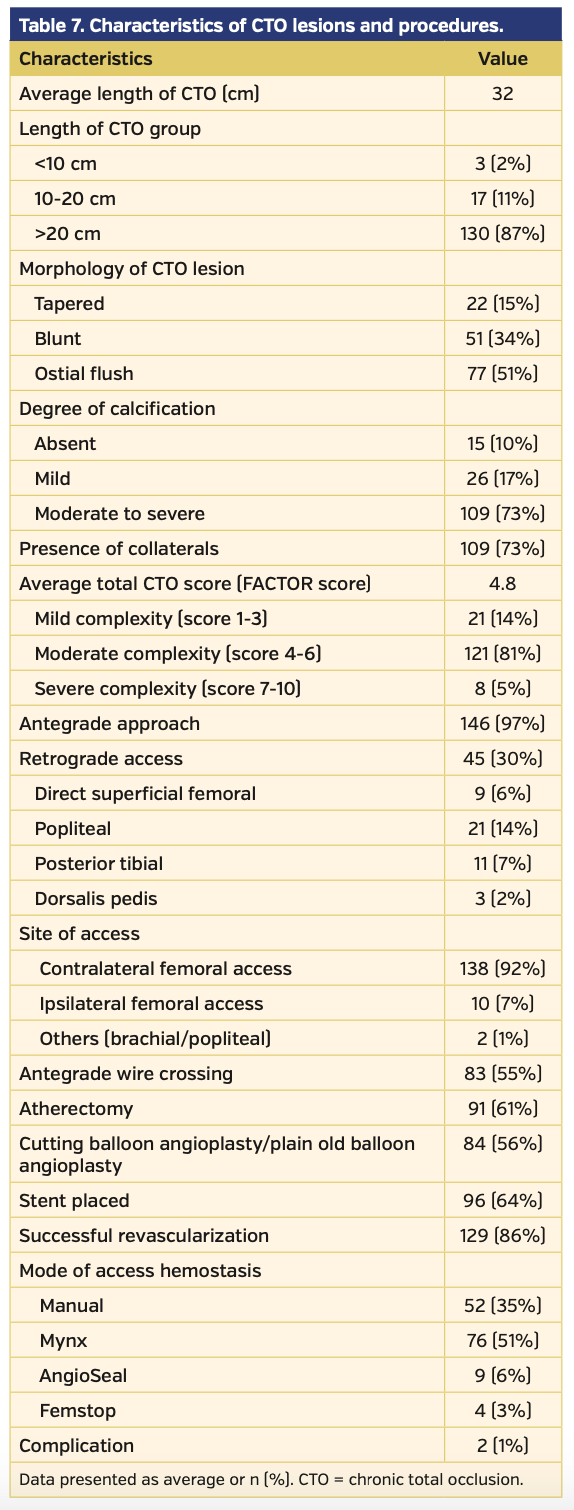
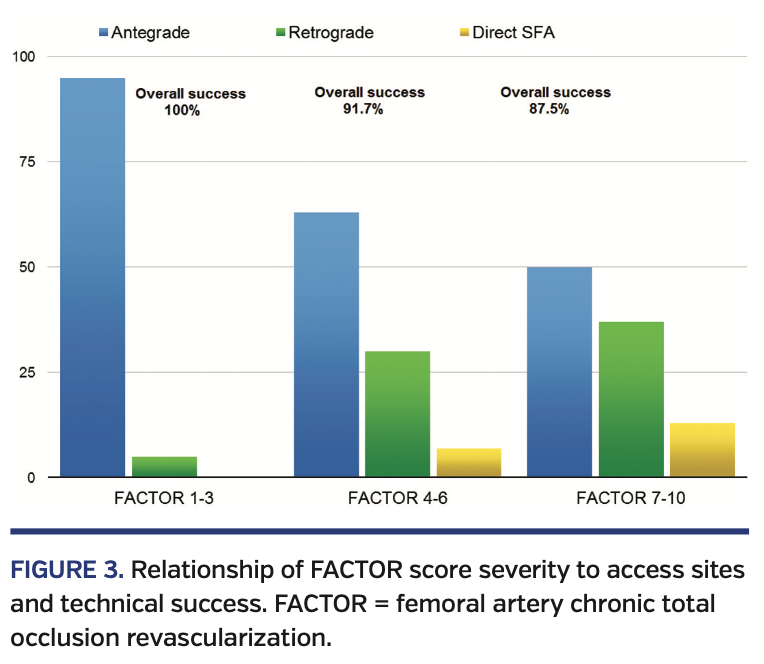
CTO lesion characteristics are summarized in Table 7. Lesions with a higher FACTOR score required additional access sites more frequently (Figure 3). Successful CTO crossing using antegrade access only was successful in 95% of all patients with scores 1-3. Retrograde popliteal artery or pedal access was used in only 5% of these patients. Patients with FACTOR scores of 4-6 required additional retrograde access in up to 30% of cases; direct SFA access was required in 7% of all cases. Finally, the most complex lesions with FACTOR scores of 7-10 were successful in antegrade crossing only 50% of the time; direct SFA access was required in 13% of cases.
Furthermore, increased operator experience with the use of FACTOR algorithm was associated with a higher technical success rate of CTO crossing over time (Figure 4). In the first 2 years following implementation of the FACTOR score and algorithm, technical success rate was 75%; the technical success rate increased to 93% in years 3 and 4. Moreover, the increased proficiency with vascular access over time resulted in a decrease for the need of repeat revascularization attempts from 14% (years 1 and 2) to 5% (years 3 and 4).
In a multivariate analysis, CTO length (odds ratio [OR], 0.005; 95% confidence interval [CI], 0.008-0.01; P=.02), retrograde wire crossing (OR, 3.4; 95% CI, 1.25-9.32; P=.02), stent placement (OR, 26.5; 95% CI, 5.9-120.0; P<.01), and atherectomy (OR, 12.2; 95% CI, 3.8-39.0; P<.01) were independent predictors of successful revascularization (Table 8). CTO length reduced the likelihood of successful CTO crossing (OR, 0.005; 95% CI, 0.008-0.01; P=.02).
Discussion
In our study of consecutive patients who underwent percutaneous revascularization of SFA-CTOs, we evaluated the use of the novel FACTOR score and algorithm on the technical success rates of CTO crossing. We found that use of the score and algorithm successfully stratified patients into low-complexity (1-3), moderate-complexity (4-6), and high-complexity lesions (7-10). In turn, the success rates of CTO crossing, particularly in the moderate- and high-complexity groups, increased significantly if alternative access points were utilized.
The field of endovascular intervention for treatment of complex PAD has expanded exponentially in recent years, making percutaneous revascularization of SFA-CTO lesions the default approach. Despite significant progress in available technology and increased physician experience, there remains a wide disparity across the country on how to best approach an SFA-CTO. We have developed the FACTOR score to allow the operator to have a better upfront assessment of lesion complexity based on specific morphologic characteristics. The preprocedural FACTOR score would in turn allow the operator to better quantify the lesion complexity and understand the likelihood of success. Having this information upfront can result in better case planning and ensure that appropriate equipment and staffing are available. In less-experienced centers, evaluation of a patient with high FACTOR score may help with arranging for a proctor to be present at the time of the procedure or even result in a transfer to a high-volume center. Moreover, the FACTOR score can further assist with improving various other quality metrics, such as reductions in procedure time, fluoroscopy time, and radiation dose.
In our study, we were able to objectively correlate the success of SFA-CTO revascularization with baseline lesion morphology. The incremental success was specifically related to the use of alternative access sites for treatment of lesions with highest complexity. Overall technical success rates increased in a stepwise fashion (from 59% ◊ 79% ◊ 85%) as additional access sites (antegrade only ◊ antegrade and retrograde ◊ antegrade, retrograde, and direct SFA) were employed. The ability to cross SFA-CTOs increased even further when patients were brought back after an initial “investment procedure.” This finding stresses the importance of operator proficiency in alternative access techniques as well as the need for flexibility to readily switch from one access point to the next.
A similar scoring system to the one outlined in the present article has been previously developed for patients undergoing percutaneous revascularization of coronary CTOs. Overall, these scores have been shown to have a significant positive impact on procedural success and efficiency.41 The coronary Japan Chronic Total Occlusion (J-CTO) grading score was developed to predict the success rate of wire crossing of coronary CTOs within a 30-minute window. The score is based on the angiographic CTO characteristics (blunt type of entry, calcification, bending, and occlusion length >20 mm) and any previous failed attempts at CTO crossing.42 The FACTOR score achieved results comparable to the well-validated J-CTO score. Similar to the J-CTO score, technical success rates in our patients declined in a stepwise fashion whenever revascularization of the most challenging lesions was attempted.
Case Examples Utilizing FACTOR Score and Algorithm
Case #1. Antegrade device crossing; device re-entry. A 58-year-old female patient with lifestyle-limiting claudication affecting the right lower extremity and corresponding abnormal ankle-brachial index (ABI) of 0.69 was brought in for peripheral angiogram and intervention. Left CFA access was obtained and a 7 Fr, 45 cm sheath inserted across the aorta-iliac bifurcation to gain access to the right CFA. Selective angiogram demonstrated complete occlusion of the proximal right SFA with reconstitution at the distal SFA (FACTOR score 3), mild popliteal artery disease, and 3-vessel runoff to the foot (Figures 5A and 5B). Device-based crossing was initially utilized with partial success, followed by wire-based crossing. Due to wire entry into the subintimal plane adjacent to the true lumen, a re-entry catheter (oriented using fluoroscopic guidance) was used to gain successful true lumen position (Figure 5C). Subsequently, balloon angioplasty and stenting were performed with excellent angiographic result with preserved distal runoff (Figure 5D).
Case #2. Retrograde tibiopedal access; CART technique. A 65-year-old male patient with lifestyle-limiting claudication affecting the left lower extremity and corresponding abnormal ABI of 0.5 was brought in for peripheral angiogram and intervention. Left CFA antegrade access was obtained and a 7 Fr sheath was inserted. Selective angiogram demonstrated occlusion of the proximal left SFA with reconstitution at the popliteal artery (FACTOR score 7), and 3-vessel runoff to the foot (Figures 6A and 6B). Device-based crossing of the SFA-CTO was initially attempted with partial crossing of the occluded segment. Wire/catheter-based crossing was subsequently used with limited success into the mid SFA.
Ultrasound-guided tibiopedal access of the left posterior tibial artery was then obtained, and device-based retrograde crossing performed to reach the mid SFA. Wire-based strategies were then attempted; however, both the antegrade and retrograde wires entered the subintimal space. Once the antegrade and retrograde wires were adjacent to each other, CART technique was used with angioplasty of the subintimal space using a 4 x 40 mm retrograde balloon (Figure 6C); after balloon inflation, the antegrade wire was successfully redirected into the true lumen and advanced into the distal vessel. Subsequent balloon angioplasty and stenting were performed with excellent angiographic result and preserved 3-vessel runoff (Figure 6D).
Case #3. Direct SFA access. A 79-year-old female with Rutherford category 3 limb ischemia of the left leg presented for peripheral angiography and revascularization of a known left SFA-CTO. Right CFA access was obtained and a 7 Fr, 45 cm sheath inserted across the aorta-iliac bifurcation to gain access to the left CFA. Selective angiogram confirmed complete occlusion of the proximal left SFA with reconstitution at the distal SFA via profunda collaterals (FACTOR score 7), severe 90% diffuse popliteal artery disease, and 2-vessel runoff to the foot with patent peroneal and anterior tibial arteries (Figures 7A and 7B). Device-based crossing was initially used with partial success, followed by wire-based techniques; however, the wire entered a subintimal plane adjacent to the true lumen in the distal SFA. Direct retrograde distal SFA access was then obtained using a stiff-angled Glidewire and placement of a 6 Fr sheath (Figure 7C). Selective angiogram of the left SFA at the access site confirmed intraluminal position with severe diffuse distal disease. The Glidewire with a support catheter was used to cross the remainder of the occlusion into the CFA. A snare was used to retrieve the retrograde access wire and externalized outside the sheath. The Glidewire was then reversed through an antegrade support catheter and used to cross the remainder of the occlusion into the distal popliteal artery. Balloon angioplasty followed by stenting was performed with good angiographic result and brisk flow to the foot (Figure 7D).
Study limitations. First, the analysis reported in this study represents a single-center experience and describes outcomes of patients who underwent revascularization by highly experienced operators; therefore, these results may not be fully generalizable across all patient subtypes and operators. Second, our study is non-randomized, and therefore we do not know how much better our algorithm would have fared against a usual approach to SFA-CTO revascularization; nonetheless, the technical success rate in our study was 93%, which is fairly high considering the challenging lesion subtype of SFA-CTO. Third, our study presents a relatively limited perspective on the use of specialized crossing devices; the study, however, is representative of the use of these devices in a real-world clinical practice.
Conclusion
We describe a new predictive model to better assess the morphologic complexity of patients undergoing percutaneous revascularization of SFA-CTOs. Using the FACTOR score and algorithm, we saw a stepwise increase in procedural success rates from 59% to 93%. The core principal of our approach is the use of alternative arterial access sites for patients with more complex lesion morphologies. Future studies will be needed to further explore the relationships between the FACTOR score, procedural times, fluoroscopy times, and radiation doses.
From the 1Division of Interventional Cardiology and Endovascular Medicine, Deborah Heart and Lung Center, Browns Mills, New Jersey; the 2Division of Cardiology, Einstein Medical Center, Philadelphia, Pennsylvania; and the 3Division of Cardiology, Temple University Hospital, Philadelphia, Pennsylvania.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Janzer reports consultant income from Avinger, Boston Scientific, and Philips. The remaining authors report no conflicts of interest regarding the content herein.
The authors report that patient consent was provided for publication of the images used herein.
Manuscript accepted June 1, 2020.
Address for correspondence: Jon C. George, MD, FACC, Director Catheterization Laboratory, Division of Interventional Cardiology and Endovascular Medicine, Einstein Medical Center, 5501 Old York Road, Philadelphia, PA 19141. Email: jcgeorgemd@hotmail.com
- Hirsch A, Haskal Z, Hertzer N, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease. Circulation. 2006;113:463-654.
- Kasapis C, Gurm HS. Current approach to the diagnosis and treatment of femoral-popliteal arterial disease. A systematic review. Curr Cardiol Rev. 2009;5:296-311.
- Murabito JM, D’Agostino RB, Silbershatz H, Wilson WF. Intermittent claudication. A risk profile from the Framingham heart study. Circulation. 1997;96:44-49.
- Boguszewski A, Torey J, Pai R, Kamalannan D, Jefic D, Davis T. Intraluminal recanalization of SFA CTOs. Endovascular Today. 2010;9:33-38.
- Norgren L, Hiatt WR, Dormandy JA, et al. Intersociety consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45:S5-S67.
- Conrad MF, Cambria RP, Stone DH, et al. Intermediate result of percutaneous endovascular therapy of femoropopliteal occlusive disease: a contemporary series. J Vasc Surg. 2006;44:762-769.
- Rabellino M, Zander T, Baldi S, et al. Clinical follow up in endovascular treatment for TASC C-D lesions in femoropopliteal segment. Catheter Cardiovasc Interv. 2009;73:701-705.
- Bodewes TC, Soden PA, Ultee KH, et al. Risk factors for 30-day unplanned readmission following infrainguinal endovascular interventions. J Vasc Surg. 2017;65:484-494.
- Sigterman TA, Bolt LJ, Snoeijs MG, et al. Radiation exposure during percutaneous transluminal angioplasty for symptomatic peripheral arterial disease. Ann Vasc Surg 2016;33:167-172.
- Bolia A, Miles KA, Brennan J, Bell PR. Percutaneous transluminal angioplasty of occlusions of the femoral and popliteal arteries by subintimal dissection. Cardiovasc Intervent Radiol. 1990;13:357-363.
- Bahia A, Lahsaei S, Bhat T, Garcia L. Navigating complex femoropopliteal and infrapopliteal anatomy in endovascular interventions: a review. J Cardiovasc Surg (Torino). 2017;58:690-697.
- Thompson CA, Jayne JE, Robb JF, et al. Retrograde techniques and the impact of operator volume on percutaneous intervention for coronary chronic total occlusions, an early US experience. JACC Cardiovasc Interv. 2009;2:834-842.
- Plaisance BR, Munir K, Share DA, et al. Safety of contemporary percutaneous peripheral arterial interventions in the elderly: insights from the BMC2 PVI registry. JACC Cardiovasc Interv. 2011;4:694-701.
- Brilakis ES, Grantham JA, Rinfret S, et al. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. JACC Cardiovasc Interv. 2012;5:367-379.
- Banerjee S, Shishehbor MH, Mustapha JA, et al. A percutaneous crossing algorithm for femoropopliteal and tibial artery chronic total occlusions (PCTO algorithm). J Invasive Cardiol. 2019;31:111-119.
- George JG, Trayer T, Kovach R. Wired for success. Guidewire escalation and techniques for successful crossing of chronic total occlusions. Endovascular Today. 2014;3(Suppl):16-19.
- Spinosa DJ, Harthun NL, Bissonette EA, et al. Subintimal arterial flossing with antegrade-retrograde intervention (SAFARI) for subintimal recanalization to treat chronic critical limb ischemia. J Vasc Interv Radiol. 2005;16:37-44.
- Chou HH, Huang HL, Hsieh CA, et al. Outcomes of endovascular therapy with the controlled antegrade retrograde subintimal tracking (CART) or reverse CART technique for long infrainguinal occlusions. J Endovasc Ther. 2016;2:330-338.
- Fusaro M, Agostoni P, Biondi-Zoccai G. “Trans-collateral” angioplasty for a challenging chronic total occlusion of the tibial vessels: a novel approach to percutaneous revascularization in critical lower limb ischemia. Catheter Cardiovasc Interv. 2008;71:268-272.
- Mossop PJ, Amukotuwa SA, Whitbourn RJ. Controlled blunt microdissection for percutaneous recanalization of lower limb arterial chronic total occlusions: a single center experience. Catheter Cardiovasc Interv. 2006;68:304-310.
- Bosiers M, Diaz-Cartelle J, Scheinert D, Peeters P, Dawkins KD. Revascularization of lower extremity chronic total occlusions with a novel intraluminal recanalization device: results of the ReOpen study. J Endovasc Ther. 2014;21:61-70.
- Selmon MR, Schwindt AG, Cawich IM, et al. Final results of the chronic total occlusion crossing with the Ocelot system II (CONNECT II) study. J Endovasc Ther. 2013;20:770-781.
- Pigott JP, Raja ML, Davis T; Connect Trial Investigators. A multicenter experience evaluating chronic total occlusion crossing with the Wildcat catheter (the CONNECT study). J Vasc Surg. 2012;56:1615-1621.
- Banerjee S, Thomas R, Sarode K, et al. Crossing of infrainguinal peripheral arterial chronic total occlusion with a blunt microdissection catheter. J Invasive Cardiol. 2014;26:363-369.
- Banerjee S, Jeon-Slaughter H, Tsai S, et al. Comparative assessment of procedure cost and outcomes between guidewire and crossing device strategies to cross peripheral artery chronic total occlusions. JACC Cardiovasc Interv. 2016;9:2243-2252.
- Gandini R, Fabiano S, Spano S, et al. Randomized control study of the Outback LTD reentry catheter versus manual reentry for the treatment of chronic total occlusions in the superficial femoral artery. Catheter Cardiovasc Interv. 2013;82:485-492.
- Scheinert D, Bräunlich S, Scheinert S, Ulrich M, Biamino G, Schmidt A. Initial clinical experience with an IVUS-guided transmembrane puncture device to facilitate recanalization of total femoral artery occlusions. EuroIntervention. 2005;1:115-119.
- Schmidt A, Keirse K, Blessing E, Langhoff R, Diaz-Cartelle J; European Study Group. Offroad re-entry catheter system for subintimal recanalization of chronic total occlusions in femoropopliteal arteries: primary safety and effectiveness results of the reroute trial. J Cardiovasc Surg (Torino). 2014;55:551-558.
- Varghese V, George JC. An innovative approach to facilitate true lumen re-entry from subintimal track in chronic total occlusions. Vascular Disease Management. 2011;8:E175-E176.
- Vatakencherry G, Gandhi R, Molloy C. Endovascular access for challenging anatomies in peripheral vascular interventions. Tech Vasc Interv Radiol. 2016;19:113-122.
- Fanelli F, Lucatelli P, Allegritti M, et al. Retrograde popliteal access in the supine patient for recanalization of the superficial femoral artery: initial results. J Endovasc Ther. 2011;18:503-509.
- Shi W, Yao Y, Wang W, et al. Combined antegrade femoral artery and retrograde popliteal artery recanalization for chronic occlusions of the superficial femoral artery. J Vasc Interv Radiol. 2014;25:1363-1368.
- Ruzsa Z, Nemes B, Bansaghi Z, et al. Transpedal access after failed anterograde recanalization of complex below the knee and femoropopliteal occlusions in critical limb ischemia. Catheter Cardiovasc Interv. 2014;83:997-1007.
- Walker CM, Mustapha J, Zeller T, et al. Tibiopedal access for crossing of infrainguinal artery occlusions: a prospective multicenter study. J Endovasc Ther. 2016;23:839-846.
- George JC. Isolated tibiopedal arterial access for minimally invasive retrograde revascularization. Vascular Disease Management. 2014;11:E87-E90.
- Rosen E, George JC. Retrograde use of re-entry catheter for revascularization of superficial femoral artery chronic total occlusion. Vascular Disease Management. 2013;10:E99-E102.
- Mustapha JA, Saab F, McGoff T, et al. Tibio-pedal arterial minimally invasive retrograde revascularization in patients with advanced peripheral vascular disease: the TAMI technique, original case series. Catheter Cardiovasc Interv. 2014;83:987-994.
- Hanna EB, Prout DL. Combined radial-pedal access strategy and radial pedal rendezvous in the revascularization of complex total occlusions of the superficial femoral artery (the “no femoral access strategy”). J Endovasc Ther. 2016;23:321-329.
- Gandini R, Del Giudice C, Assako Ondo EP, et al. Stent puncture for recanalization of occluded superficial femoral artery stents. J Endovasc Ther. 2012;19:30-33.
- Schmidt A, Bausback Y, Piorkowski M, et al. Retrograde cannulation technique for use after failed antegrade angioplasty in chronic femoral artery occlusions. J Endovasc Ther. 2012;19:23-29.
- Christopoulos G, Wyman RM, Alaswad K, et al. Clinical utility of the Japan-chronic total occlusion score in coronary chronic total occlusion interventions: results from a multicenter registry. Circ Cardiovasc Interv. 2015;8:e002171.
- Morino Y, Abe M, Morimoto T, et al; Investigators JCR. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J-CTO (multicenter CTO registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011;4:213-221.