Embolic Protection Device Use and Outcomes in Patients Receiving Saphenous Vein Graft Interventions — A Single-Center Experience
Abstract: Background. Percutaneous treatment of saphenous vein graft disease is hampered by high rates of periprocedural myocardial infarction (MI). The use of embolic protection devices (EPD) during these interventions is a class IB recommendation when technically feasible, yet they are used routinely in less than half of all cases. Our aim was to explore whether or not the under-utilization of EPDs led to any untoward cardiovascular events. Methods. Consecutive vein graft interventions from 2003-2008 were identified and stratified by EPD use. Information pertaining to demographics, comorbidities, medication use, and procedural details was collected. Primary endpoint was to compare the incidence of periprocedural MI, defined as any creatinine kinase-MB fraction elevation greater than 3 times the upper limit of normal between patients who underwent percutaneous coronary intervention (PCI) for saphenous vein grafts (SVG) with EPD versus patients who underwent PCI for SVG without EPD. Secondary endpoints included comparison of the incidence of recurrent ischemia, MI, revascularization (PCI or coronary artery bypass graft [CABG]), and mortality in the above 2 groups by the end of 1 year. Results. A total of 164 consecutive vein graft interventions were identified. EPDs were used in 71 cases (43.4%). The EPD group was further out since their CABG and had a higher prevalence of hypertension and diabetes. Periprocedural MI occurred in 22 cases; 12 in the non-EPD group and 10 in the EPD group (14.1 vs 12.9%; P=.82). The composite endpoint of death, MI, or target vessel revascularization at 12 months was significantly lower when EPDs were used (11.3 vs 25.8%; P=.03). On multivariate analysis, chronic kidney disease increased the risk of periprocedural MI (odds ratio [OR], 5.36; 95% confidence interval [CI], 1.90-15.13; P=.002), whereas the use of beta-blockers was protective (OR, 0.22; 95% CI, 0.07-0.70; P=.011). Conclusions. EPD use during vein graft interventions did not improve periprocedural MI rates. However, the composite endpoint of adverse cardiovascular outcomes at 1 year was significantly reduced. EPDs are used in a minority of vein graft interventions. Efforts aimed at improving adherence to EPD use may improve long-term outcomes, though this hypothesis should be tested using prospective, randomized studies.
J INVASIVE CARDIOL 2012;24(1):1-3
Key words: embolic protection device, saphenous vein graft intervention
__________________________________________
Coronary artery bypass grafting (CABG) remains the standard of care for selected patients with left main and complex multivessel coronary artery disease. Up to 20% of saphenous vein grafts (SVG) fail within 2 years of CABG.1 The probability of graft degeneration increases with time, and by 10 years approximately 40% of SVGs are occluded and 75% develop severe narrowing.2-4 Treatment of vein graft lesions is accomplished by repeat CABG or percutaneous coronary intervention (PCI). Repeat operation carries an increased risk of morbidity and mortality; therefore, PCI is generally more commonly utilized for SVG lesions.5 It is well known that PCI of SVGs is associated with an increased risk of complications relative to native coronary PCI.6 In an effort to reduce the incidence of periprocedural myocardial infarction (MI) as well as to reduce other major adverse cardiac events after SVG-PCI, embolic protection devices (EPD) have been used. These devices are designed to minimize distal embolization of debris during angioplasty and stenting of SVG lesions. To date, only a few randomized controlled trials establishing the efficacy of EPDs have been published. One of these, the SAFER trial, demonstrated reductions in no-reflow and 30-day MI with EPD use.2 As a result, current American College of Cardiology/American Heart Association guidelines recommend EPD use in all SVG interventions when technically feasible.4
Innovations in interventional techniques, devices, antiplatelet drugs, and anticoagulation strategies may have reduced periprocedural events in the modern era. We therefore sought to conduct a retrospective study to determine the efficacy of EPD in reducing the risk of periprocedural MI, as well as to determine the incidence of recurrent ischemia, MI, revascularization, and mortality at 1 year.
Methods
 Consecutive vein graft interventions from 2003-2008 at Oklahoma City Veteran Affairs Medical Center were identified and stratified by EPD use. Information pertaining to demographics, comorbidities, and medication use was collected. The primary endpoint was to compare the incidence of periprocedural MI, defined as any creatinine kinase-MB fraction elevation greater than 3 times the upper limit of normal (3X ULN) between patients who underwent PCI for SVGs with EPD versus patients who underwent PCI for SVG without EPD. Secondary endpoints included comparison of the incidence of recurrent ischemia, MI, revascularization (PCI or CABG), and mortality in the above 2 groups by the end of 1 year.
Consecutive vein graft interventions from 2003-2008 at Oklahoma City Veteran Affairs Medical Center were identified and stratified by EPD use. Information pertaining to demographics, comorbidities, and medication use was collected. The primary endpoint was to compare the incidence of periprocedural MI, defined as any creatinine kinase-MB fraction elevation greater than 3 times the upper limit of normal (3X ULN) between patients who underwent PCI for SVGs with EPD versus patients who underwent PCI for SVG without EPD. Secondary endpoints included comparison of the incidence of recurrent ischemia, MI, revascularization (PCI or CABG), and mortality in the above 2 groups by the end of 1 year.
Values are expressed as means ± standard deviations, where applicable. Categorical variables were analyzed using Fisher’s exact test and continuous variables were analyzed with unpaired t-testing. Multivariate logistic regression analysis was employed to assess for independent predictors of periprocedural MI.
Results
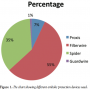
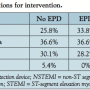
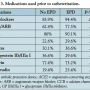
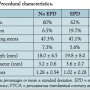
 Patient characteristics. A total of 164 consecutive vein graft interventions were identified. EPDs were used in 71 cases (43.4%). Demographics and comorbidities are shown in Table 1. The EPD group had a higher prevalence of hypertension and diabetes. Time since CABG was significantly longer in the EPD group as well. Table 2 shows that the indications for intervention in both groups were similar; however, EPD was not used in any patient with ST-segment elevation MI. Table 3 shows the medications prior to catheterization. The EPD group had more patients on beta-blockers and ACE inhibitors as compared to the non-EPD group. More importantly, however, the anticoagulation and antiplatelet strategies between the 2 groups (bivaliridin, heparin, and glycoprotein IIb/IIIa inhibitors) were similar. Procedural characteristics are presented in Table 4. The different types of embolic protection devices used are depicted in Figure 1.
Patient characteristics. A total of 164 consecutive vein graft interventions were identified. EPDs were used in 71 cases (43.4%). Demographics and comorbidities are shown in Table 1. The EPD group had a higher prevalence of hypertension and diabetes. Time since CABG was significantly longer in the EPD group as well. Table 2 shows that the indications for intervention in both groups were similar; however, EPD was not used in any patient with ST-segment elevation MI. Table 3 shows the medications prior to catheterization. The EPD group had more patients on beta-blockers and ACE inhibitors as compared to the non-EPD group. More importantly, however, the anticoagulation and antiplatelet strategies between the 2 groups (bivaliridin, heparin, and glycoprotein IIb/IIIa inhibitors) were similar. Procedural characteristics are presented in Table 4. The different types of embolic protection devices used are depicted in Figure 1.
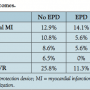
 Outcomes. The primary endpoint of the study, which was periprocedural MI as defined above, occurred in 22 cases — 12 in the non-EPD group and 10 in the EPD group (14.1 vs 12.9%; P=.82). In addition, when analysis was done using any troponin elevation as a marker for periprocedural MI, there was no statistical difference between the 2 groups. Based on the multivariate logistic regression model, independent predictors of periprocedural MI included chronic kidney disease, which increased the risk by approximately 5 times (odds ratio [OR], 5.36; 95% confidence interval [CI], 1.90-15.13; P=.002), whereas the use of beta-blockers was protective (OR, 0.22; 95% CI, 0.07-0.70; P=.011).
Outcomes. The primary endpoint of the study, which was periprocedural MI as defined above, occurred in 22 cases — 12 in the non-EPD group and 10 in the EPD group (14.1 vs 12.9%; P=.82). In addition, when analysis was done using any troponin elevation as a marker for periprocedural MI, there was no statistical difference between the 2 groups. Based on the multivariate logistic regression model, independent predictors of periprocedural MI included chronic kidney disease, which increased the risk by approximately 5 times (odds ratio [OR], 5.36; 95% confidence interval [CI], 1.90-15.13; P=.002), whereas the use of beta-blockers was protective (OR, 0.22; 95% CI, 0.07-0.70; P=.011).
 The secondary endpoints of the study, which included the composite endpoint of death, MI, or TVR at 12 months, were significantly lower when EPDs were used (11.3 vs 25.8%; P=.03) (Table 5). EPD use independently predicted a lower risk of the combined endpoint of death, MI, or TVR at 12 months (OR, 0.26; 95% CI, 0.10-0.73; P=.010) by multivariate logistic regression analysis. In the same model, statin use (OR, 0.15; 95% CI, 0.04-0.51; P=.003) and bivalirudin use (OR, 0.17; 95% CI, 0.03-0.95; P=.043) were associated with a decreased risk of the combined endpoint, whereas diabetes mellitus (OR, 5.61; 95% CI, 1.72-18.25; P=.004) and non-ST elevation MI as an indication for PCI (OR, 3.19; 95% CI, 1.24-8.23; P=.016) increased the risk of the combined endpoint.
The secondary endpoints of the study, which included the composite endpoint of death, MI, or TVR at 12 months, were significantly lower when EPDs were used (11.3 vs 25.8%; P=.03) (Table 5). EPD use independently predicted a lower risk of the combined endpoint of death, MI, or TVR at 12 months (OR, 0.26; 95% CI, 0.10-0.73; P=.010) by multivariate logistic regression analysis. In the same model, statin use (OR, 0.15; 95% CI, 0.04-0.51; P=.003) and bivalirudin use (OR, 0.17; 95% CI, 0.03-0.95; P=.043) were associated with a decreased risk of the combined endpoint, whereas diabetes mellitus (OR, 5.61; 95% CI, 1.72-18.25; P=.004) and non-ST elevation MI as an indication for PCI (OR, 3.19; 95% CI, 1.24-8.23; P=.016) increased the risk of the combined endpoint.
Discussion
 In our experience, EPD use during vein graft interventions did not improve periprocedural MI rates, but a composite endpoint of adverse cardiovascular outcomes at 1 year was significantly reduced (driven by TVR). In addition, chronic renal insufficiency increased the risk of periprocedural MI 5 times, which is similar to conclusions drawn by Osten et al.7 Despite newer antiplatelet and anticoagulation strategies, the use of EPDs in SVG-PCI continues to show benefit to reduce adverse cardiovascular outcomes at the end of 1 year.
In our experience, EPD use during vein graft interventions did not improve periprocedural MI rates, but a composite endpoint of adverse cardiovascular outcomes at 1 year was significantly reduced (driven by TVR). In addition, chronic renal insufficiency increased the risk of periprocedural MI 5 times, which is similar to conclusions drawn by Osten et al.7 Despite newer antiplatelet and anticoagulation strategies, the use of EPDs in SVG-PCI continues to show benefit to reduce adverse cardiovascular outcomes at the end of 1 year.
 EPDs remain under-utilized, which is consistent with previous reports. Mehta et al analyzed 19,546 SVG-PCI procedures from the ACC-NCDR registry and concluded that EPDs are used in <25% of procedures.8 Reasons for under-utilization may include technical considerations, lesion complexity, operator uncertainty or inexperience, and concerns about cost-effectiveness. The cost-effectiveness analysis for usage of EPDs as conducted in the SAFER trial concluded that EPDs increase initial procedural costs by approximately $1600; however, by reducing ischemic complications, distal protection reduced mean hospital costs by nearly $1000, and thus overall initial hospital costs were only $582 per patient higher with distal protection.11 Similar conclusions were drawn by Senter et al in their analysis of 126 consecutive patients.12
EPDs remain under-utilized, which is consistent with previous reports. Mehta et al analyzed 19,546 SVG-PCI procedures from the ACC-NCDR registry and concluded that EPDs are used in <25% of procedures.8 Reasons for under-utilization may include technical considerations, lesion complexity, operator uncertainty or inexperience, and concerns about cost-effectiveness. The cost-effectiveness analysis for usage of EPDs as conducted in the SAFER trial concluded that EPDs increase initial procedural costs by approximately $1600; however, by reducing ischemic complications, distal protection reduced mean hospital costs by nearly $1000, and thus overall initial hospital costs were only $582 per patient higher with distal protection.11 Similar conclusions were drawn by Senter et al in their analysis of 126 consecutive patients.12
Limitations. The limitations of this study primarily relate to its retrospective design. An angiographic analysis was not performed due to unavailability of all the angiographic data (ie, degeneration score and reference diameter as well as stent-to-SVG diameter ratio) and as such, differences in clinical endpoints may be a result of the “selection” of patients with a lower burden of atherosclerotic disease. Likewise, differences in operator technique may have influenced outcomes (eg, concurrent pharmaco-protection with vasodilators with or without EPD use).
Conclusion
In conclusion, EPDs are used in less than 50% of vein graft interventions. Though no reduction in periprocedural MI was shown here, EPD use was associated with significant reductions in cardiovascular endpoints at 1 year. Efforts aimed at improving adherence to EPD use may improve long-term outcomes, though this hypothesis should be tested using prospective, randomized studies.
References
- Savage MP, Douglas JS Jr, Fischman DL, et al. Stent placement compared with balloon angioplasty for obstructed coronary bypass grafts. Saphenous Vein De Novo Trial Investigators. N Engl J Med. 1997;337(11):740-747.
- Baim DS, Wahr D, George B, et al. Randomized trial of a distal embolic protection device during percutaneous intervention of saphenous vein aorto-coronary bypass grafts. Circulation. 2002;105(11):1285-1290.
- Singh M, Rihal CS, Gersh BJ, et al. Twenty-five-year trends in in-hospital and long-term outcome after percutaneous coronary intervention: a single-institution experience. Circulation. 2007;115(22):2835-2841.
- Smith SC Jr, Feldman TE, Hirshfeld JW Jr, et al. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention — summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). Circulation. 2006;113(1):156-175.
- Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5065 grafts related to survival and reoperation in 1388 patients during 25 years. J Am Coll Cardiol. 1996;28(3):616-626.
- Piana RN, Paik GY, Moscucci M, et al. Incidence and treatment of ‘no-reflow’ after percutaneous coronary intervention. Circulation. 1994;89(6):2514-2518.
- Osten MD, Ivanov J, Eichhofer J, et al. Impact of renal insufficiency on angiographic, procedural, and in-hospital outcomes following percutaneous coronary intervention. Am J Cardiol. 2008;101(6):780-785.
- Mehta SK, Frutkin AD, Milford-Beland S, et al. Utilization of distal embolic protection in saphenous vein graft interventions (an analysis of 19,546 patients in the American College of Cardiology-National Cardiovascular Data Registry). Am J Cardiol. 2007;100(7):1114-1118.
- Lim MJ, Young JJ, Senter SR, Klein LW. Determinants of embolic protection device use: case study in the acceptance of a new medical technology. Catheter Cardiovasc Interv. 2005;65(4):597-599.
- Mahmood A, Khair T, Abdel-Karim AR, et al. Contemporary approaches to saphenous vein graft interventions: a survey of 275 interventional cardiologists. Catheter Cardiovasc Interv. 2011 Apr 28. (Epub ahead of print).
- Cohen DJ, Murphy SA, Baim DS, et al. Cost-effectiveness of distal embolic protection for patients undergoing percutaneous intervention of saphenous vein bypass grafts: results from the SAFER trial. J Am Coll Cardiol. 2004;44(9):1801-1808.
- Senter SR, Nathan S, Gupta A, Klein LW. Clinical and economic outcomes of embolic complications and strategies for distal embolic protection during percutaneous coronary intervention in saphenous vein grafts. J Invasive Cardiol. 2006;18(2):49-53.
__________________________________________
From the 1Department of Internal Medicine, 2Cardiovascular Section and 3Heart Rhythm Institute, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted May 17, 2011, provisional acceptance given June 27, 2011, final version accepted October 16, 2011.
Address for correspondence: Mazen Abu-Fadel, MD, Section of Cardiovascular Diseases, Department of Internal Medicine, 920 Stanton L. Young Boulevard, WP 3010, Oklahoma City, OK 73104. Email: Mazen-Abufadel@ouhsc.edu
















