The Efficacy of Percutaneous Transluminal Angioplasty on the Limb Salvage and Recovery of Symptoms in Patients With Buerger’s Disease With Critical Limb Ischemia
Abstract: Introduction. Buerger’s disease, or thromboangiitis obliterans, is associated with limb-threatening chronic arterial lesions. In this study, we sought to investigate the efficacy of the percutaneous transluminal angioplasty method for the treatment of critical limb ischemia (CLI) in patients with Buerger’s disease in our modest cohort. Methods. Patients diagnosed with CLI secondary to Buerger’s disease who underwent percutaneous transluminal angioplasty between May 2014 and June 2017 were retrospectively investigated. Patient demographics, presentations, procedural details, responses to percutaneous treatment, complications, limb salvage, wound healing, reinterventions, and early follow-up data were recorded. Results. The cohort included 24 patients with Buerger’s disease presenting with CLI observed in 46 limbs. Presentations were gangrene in 12 patients, ulcer formation in 7 patients, and rest pain in the remaining 5 patients. All patients received percutaneous balloon angioplasty, with limb salvage in 21 patients (87.5%). Revascularization was achieved in 87.5% of the destination arteries at the primary intervention and overall technical success rate including reinterventions reached 95.8%. Following the procedures, a total of 22 patients had clinical response with at least ≥1 Rutherford category and mean Rutherford category significantly improved from 5.2 ± 0.74 to 1.6 ± 0.7 (P<.001). Limb salvage rate was 87.5%. Complete wound healing was achieved in all patients with ischemic ulcers at 3.9 ± 2.6 months (range 1–13 months) post revascularization. Mean follow-up duration was 16.07 ± 3.4 months and 6 patients (who were especially subjected to cigarette smoke) required reinterventions. Conclusion. Percutaneous treatment of arterial occlusions in patients with Buerger’s disease seems feasible in the current era of improving devices and angioplasty materials. Procedures may be safely performed with good technical and clinical success rates, and without mortality or complications as experience increases.
Key words: angioplasty, Buerger’s disease, critical limb ischemia, endovascular, thromboangiitis obliterans, treatment
Buerger’s disease, or thromboangiitis obliterans, is a chronic inflammatory arteritis without prominent evidence of atherosclerosis.1 Pathology was first described by Von Winiwarter in 1879 in a young, actively smoking male patient.2 Leo Buerger described the histopathological findings of the disease in detail in 11 amputated limbs in 1908.3
Buerger’s disease is usually a non-atherosclerotic and inflammatory process affecting the small- and medium-sized arteries, veins, and nerves of the arms and legs. It leads to thrombotic occlusion in the distal vasculature of the extremities. The pathology most commonly affects male smokers at young ages. Buerger’s disease may be seen worldwide; however, the prevalence is higher in the Middle East and Far East populations when compared with the West.4
Patients are usually asymptomatic until the advanced stages of the disease. The presentation is usually with ischemic symptoms in the extremities, including claudication, rest pain, or ulcer/gangrene secondary to the occlusion of the distal arteries and veins. Symptoms are generally poorly controlled and claudication may rapidly progress to ischemic pain and distal gangrene leading to limb loss.3 Rates of major amputation have been reported in increased percentages in actively smoking patients without treatment.5
The diagnosis of Buerger’s disease is mainly based on clinical findings and association with cigarette smoking. Certain diagnostic criteria were established, and include history of tobacco exposure, initiation of the symptoms before the age of 50 years, the presence of distal-extremity ischemia without evidence of trauma, embolism, atherosclerosis, hypercoagulable state, or an autoimmune disorder.4
As previously stated, tobacco exposure is the major factor in the pathogenesis of Buerger’s disease and treatment of patients must start with cessation of smoking. On the other hand, in symptomatic patients and especially in patients with extremity-threatening arterial occlusions, additional invasive and medical measures are usually required; otherwise extremity loss may occur. The aim should be prompt restoration of blood flow to the extremities with available medical and invasive tools.5,6
Together with medical and technological advances, endovascular therapeutic options for the treatment of vaso-occlusive disorders have evolved tremendously, with various procedural and long-term success rates in certain conditions. Historically, endovascular treatment strategies in the treatment of Buerger’s disease have been disappointing, most likely due to the diffuse inflammatory process throughout the vessels. Moreover, endovascular revascularization in Buerger’s disease has not been investigated in detail in the current era.5
In this study, we sought to investigate the efficacy of percutaneous revascularization of diseased arteries in symptomatic patients with Buerger’s disease.
Methods
Between May 2014 and June 2017, a total of 24 patients with Buerger’s disease and CLI with tissue loss or rest pain involving the lower-extremity arteries in 46 limbs underwent percutaneous transluminal angioplasty (PTA), and comprise the study population. The patients were evaluated in the context of CLI for duration of symptoms, gender, medical therapy, clinical manifestations, symptoms, procedure success, response to treatment, wound healing, amputation, and early- and long-term outcomes. This study was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. Retrospective analyses of patient data were performed following retrieval of the recorded data from our institutional files.
Shionoya criteria were used for the clinical diagnoses. The criteria included presentation before 50 years of age, smoking history, infrapopliteal peripheral arterial disease, phlebitis migrans or involvement of upper-limb disease, and only the presence of smoking as atherosclerosis risk factor.7 The history and physical examinations, including ankle-brachial indexes (ABIs), were evaluated, and Doppler ultrasonography, computed tomography angiography, and/or digital-subtraction angiography scans were analyzed. Rutherford category (0 = asymptomatic; 1 = mild claudication; 2 = moderate claudication; 3 = severe claudication; 4 = rest pain; 5 = minor tissue loss with non-healing ulcer or focal gangrene; and 6 = major tissue loss extending above the transmetatarsal level)8 was used before and after the intervention.
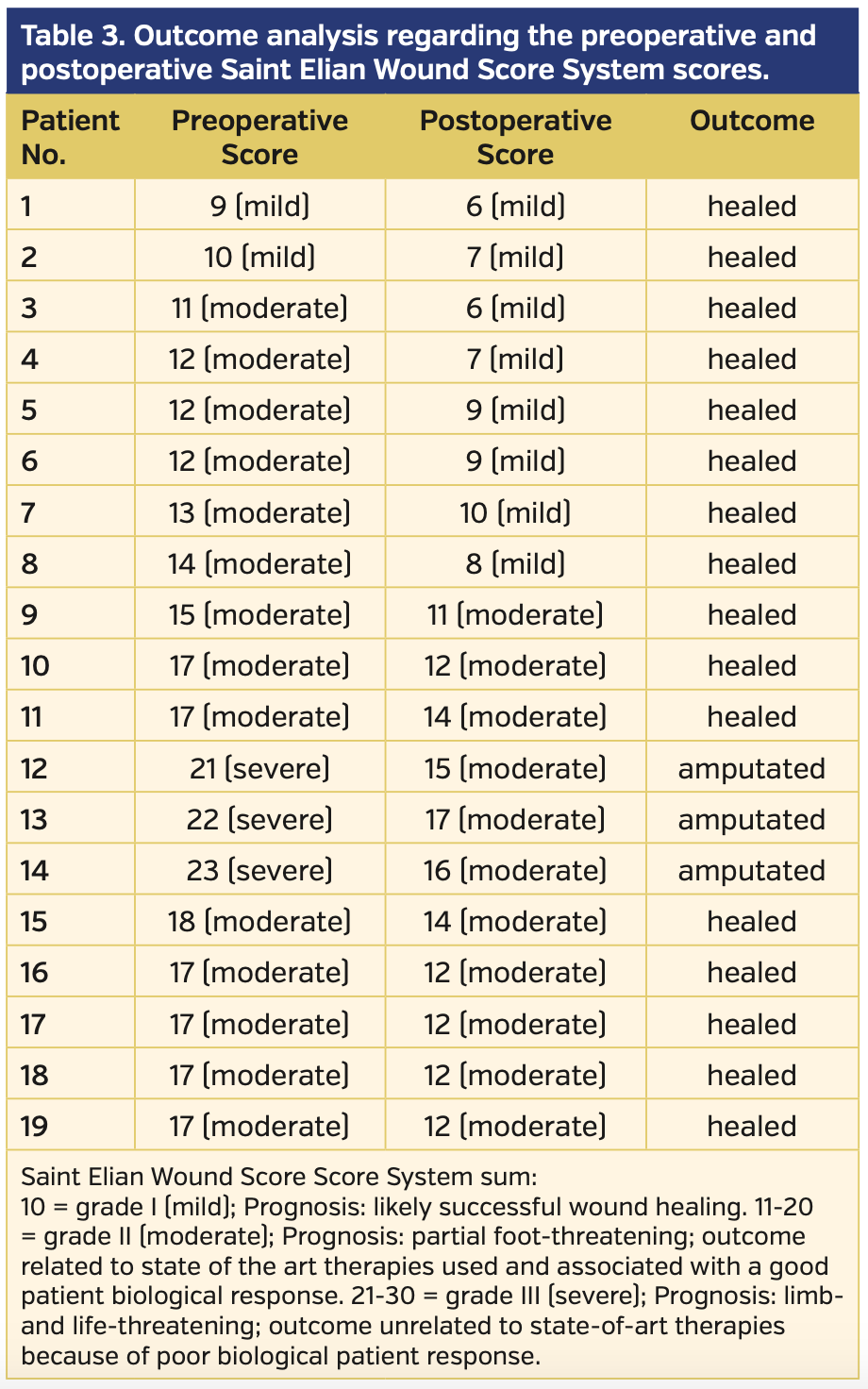
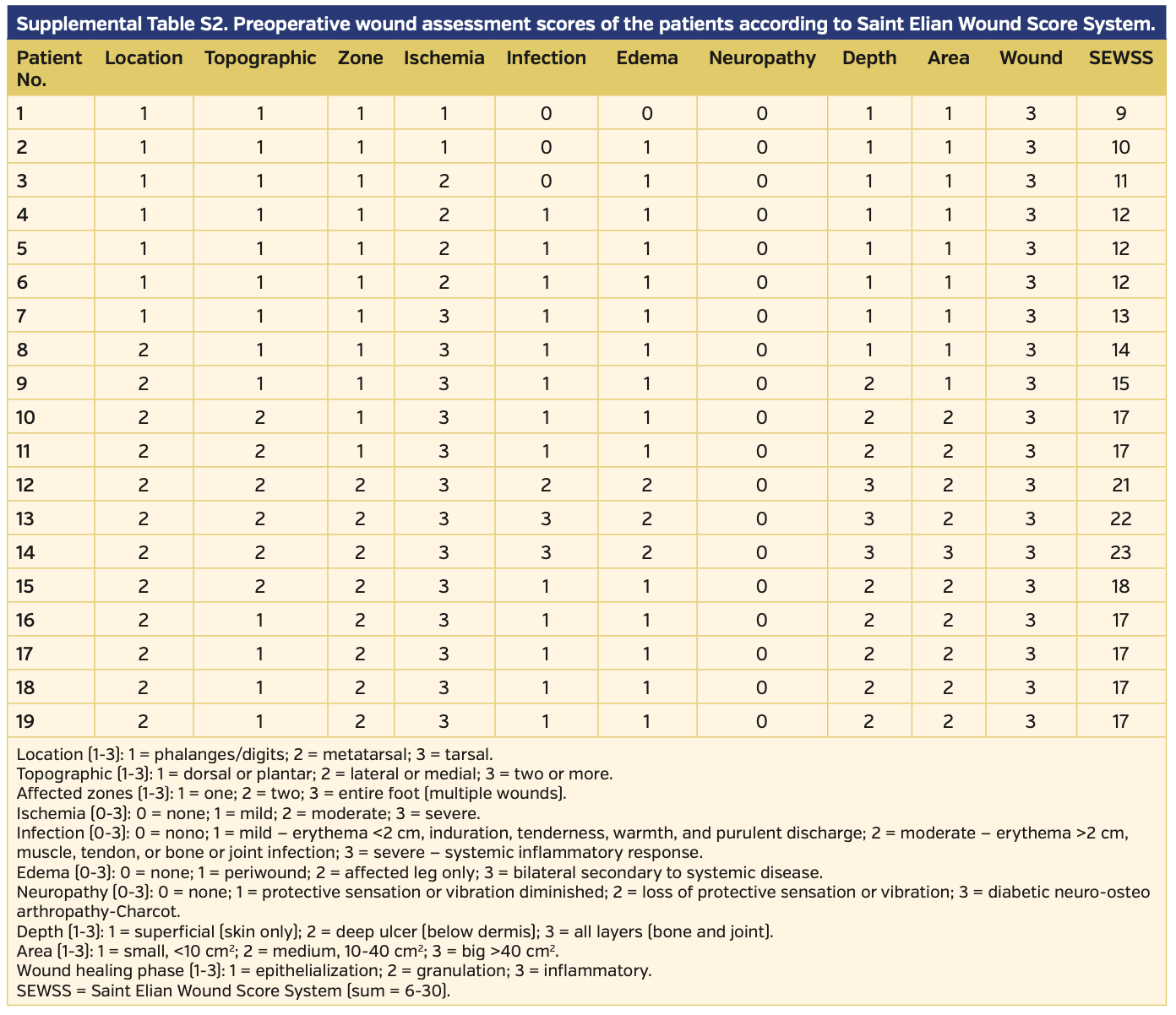
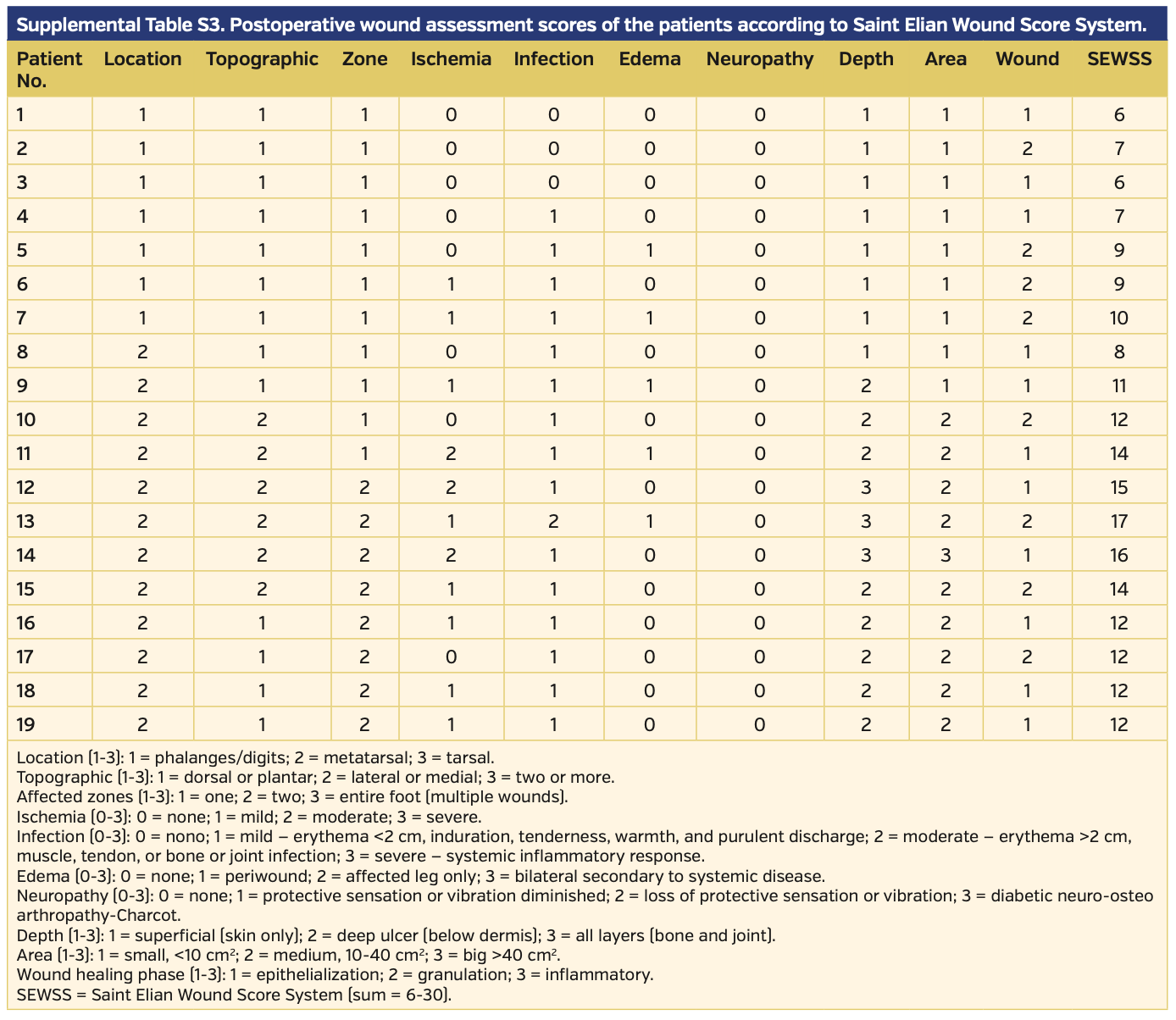
Endovascular revascularization was planned when ABI and skin perfusion pressure (SPP) measurements indicated CLI, which was defined as lower-limb critical ischemia including tissue loss and SPP <40 mm Hg. Procedural success was defined as the revascularization of affected vessels with residual stenosis <30% for femoropopliteal lesions and at least 1 recanalization to the foot with residual stenosis <30% for infrapopliteal lesions; increase in ABI and SPP were also accepted as early success. Revascularization procedures, relief of symptoms, healing of infections and gangrene, and amputations were recorded. The Saint Elian Wound Score System (SEWSS)9 categorizes 10 wound variables from mild to moderate and severe, and the final score grades severity as I, II, or III; this system was used for the classification of each limb and for further assessment of the wounds, infections, or ischemia during initial evaluation, as well as during patient follow-up for reintervention requirement, amputation, or stenosis.
Wound management utilized a multidisciplinary team approach including orthopedicians, cardiovascular surgeons, and plastic surgeons. Infections were treated with antibiotics, wounds were cleaned with debridements, and minor amputations were performed when appropriate. Rehabilitation was also provided when required. In the clinical assessment, patients were seen at 1 week post intervention, then monthly for 3 months. Thereafter, follow-up visits occurred every 3 months. During the follow-up period, recurrent symptoms, major or minor amputations, and other events were investigated.
Smoking control was of the utmost importance and definite cigarette and smoking control was provided. All patients who confessed they might not be able to obey strict smoking control were consulted by psychologists. In addition, these patients were referred to chest disease clinics for free access to varenicline (Pfizer) use for 12 weeks for smoking cessation, which is a free service provided by the health system in Turkey. In addition to strict cigarette control, abstinence from all other nicotine products, including pipe, cigar, or nicotine patches, was strongly advised.
Procedures. The procedures were performed with local anesthesia and intravenous sedation. General anesthesia was required intraoperatively due to severe lower-limb pain in 1 patient who underwent Syme amputation. Femoral sheaths were inserted for vascular access and a bolus dose of 5000 U intra-arterial heparin was given. Although ipsilateral antegrade approach was routine in our procedures, patients with proximal lesions were accessed contralaterally with a crossover technique.
For the treatment of femoropopliteal lesions, 0.035˝ guidewires were preferred. In cases of total occlusion, 5 Fr x 135 cm Navicross micro-occlusion catheters (Terumo) were used and PTA was performed. Biopath drug-coated balloons in diameters of 4-6 mm (Biosensors) were used in all patients. While 0.014˝ guidewires were used in the infrapopliteal target vessels, long balloons with 2-3 mm diameters were used for tibial arteries. Shorter, 1.25-2.5 mm diameter balloons were used for pedal arteries, along with 2-4 minute inflations.
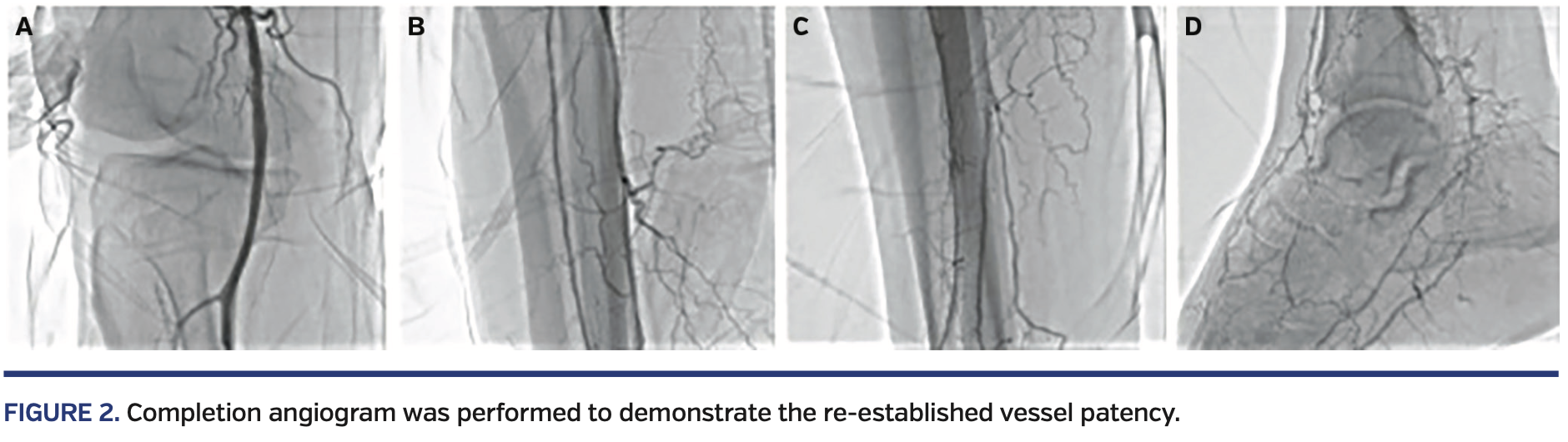
Although the aims, revascularizations, and target vessels were determined depending on the angiosome distributions of the wound or ulcer, all feasible infrapopliteal arteries were treated extensively. Examples of infrapopliteal and femoropopliteal revascularizations are shown in Figure 1. Completion angiograms were performed to evaluate the procedural success, with an example shown in Figure 2.
Medical therapy comprised 300 mg aspirin or 300 mg clopidogrel as loading doses 12 hours before the intervention. The patients also received 600 mg clopidogrel following the intervention. Medical therapy continued with anticoagulation with intravenous standard heparin, with an aim to keep the activated partial thromboplastin time 1.5 times the normal values for 24 hours following the intervention. At the time of discharge, a dual-antiplatelet regimen with aspirin (100 mg/day) and clopidogrel (75 mg/day) was initiated.
Statistical analysis. The Statistical Package for Social Sciences, version 20 for Windows (SPSS) was used for statistical analyses. Conformity of normal distribution and homogeneity were tested with the Kolmogorov-Smirnov test for the analysis of continuous variables. Categorical values were evaluated with Chi-Square test, and parametric values were evaluated with the independent samples t-test. Findings are presented as either mean value ± standard deviation or as percentages. Mann-Whitney U-test was used to compare the clinical assessment of ischemia at the end of follow-up and Fischer’s Exact test was used to compare reintervention requirement between smokers and non-smokers. A P-value <.05 was considered statistically significant.
Results
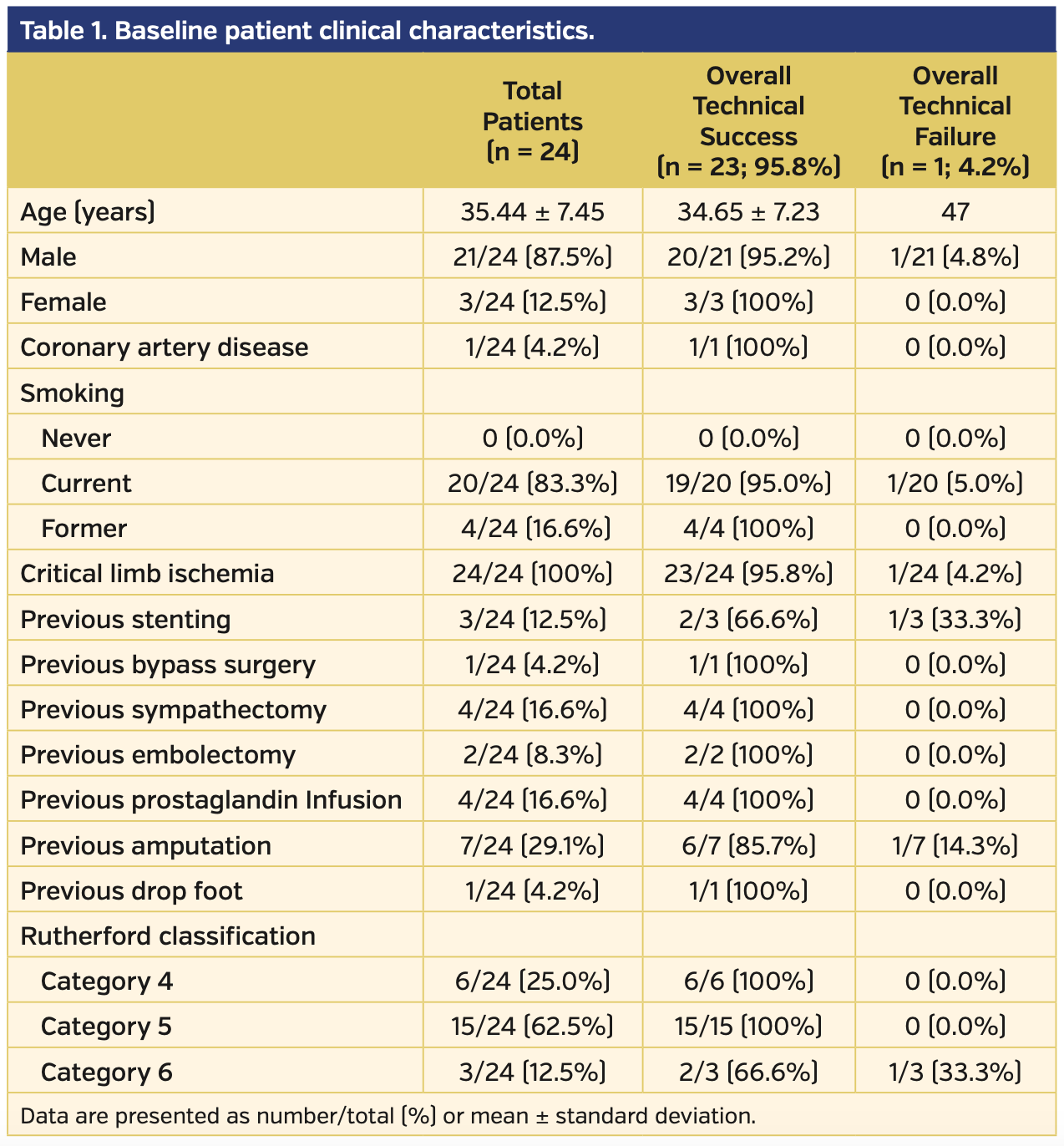
A total of 24 patients with Buerger’s disease diagnosis were examined retrospectively. The patient diagnoses relied upon Shionoya criteria as well as radiographic findings, such as corkscrew appearance of arteries and spiral collaterals (Figures 1 and 2) with relatively protected proximal medium- to large-sized arteries. All patients had CLI in the lower extremity and were treated with endovascular interventions. Baseline clinical characteristics and previous interventions of the patients are summarized in Table I.
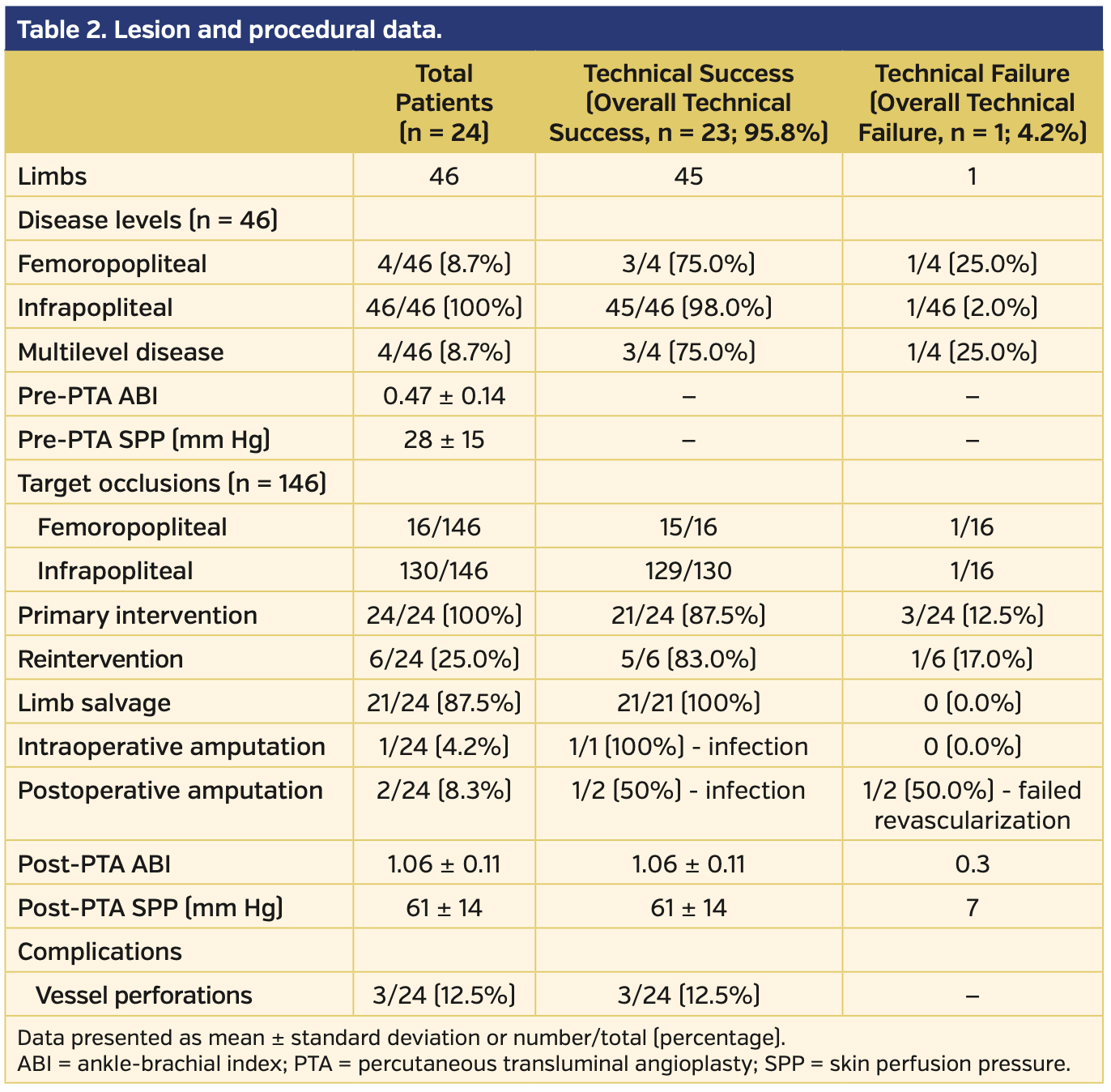
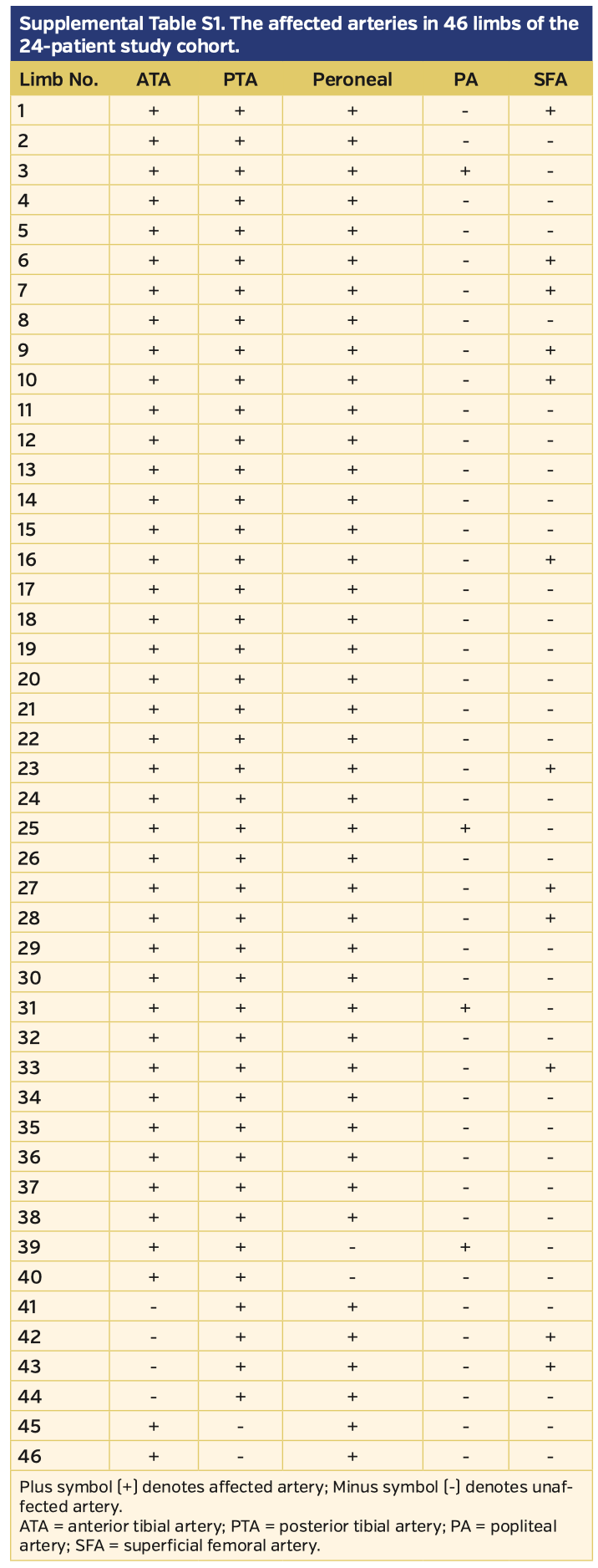
Three patients were female and 21 were male. All patients were smokers (20 current and 4 former). Mean patient age was 35.44 ± 7.45 years. Five patients presented to the clinic with ischemic rest pain and the remaining 19 presented with wounds (7 patients had ulcers and 12 patients had gangrenous lesions). Among the 24 patients, a total of 46 limbs and 146 total occlusions (8 superficial femoral artery, 8 popliteal artery, 37 peroneal artery or tibioperoneal trunk, 41 anterior tibial artery, 40 posterior tibial artery, and 12 plantar arteries) were intervened with PTA. Lesion and procedural data are summarized in Table 2. The angiographic details regarding the affected extremities are presented in Supplemental Table S1 (supplemental materials available at www.invasivecardiology.com). The technical success rate of the primary intervention was 87.5% (n = 21 patients) and 82.8% of arteries. There was no mortality or morbidity during the procedures. Following reinterventions and medical therapy, the technical success rate increased to 95.8% (n = 23).
The wounds of 19 patients were categorized according to the SEWSS before and after the interventions (Supplemental Tables S1 and S2, respectively). The mean score on admission to the clinic was 15.47 ± 3.99 (range, 9-23) and decreased to 11.0 ± 3.33 (range, 6-17) post intervention. The outcome analysis regarding SEWSS for each patient is presented on Table 3. The decrease in SEWSS was statistically significant (P<.001).
One female and 2 male patients underwent amputation due to infection despite successful revascularization. In addition to the patient who underwent PTA and received Syme amputation at the same session with general anesthesia, an- other Syme amputation was performed in a second patient on day 17 post intervention. Only 1 patient with failed reintervention despite primary successful revascularization underwent a major below-the-knee amputation due to infectious necrosis and open foot wound at 8 months post PTA.Total limb salvage rate was 87.5% (n = 21).
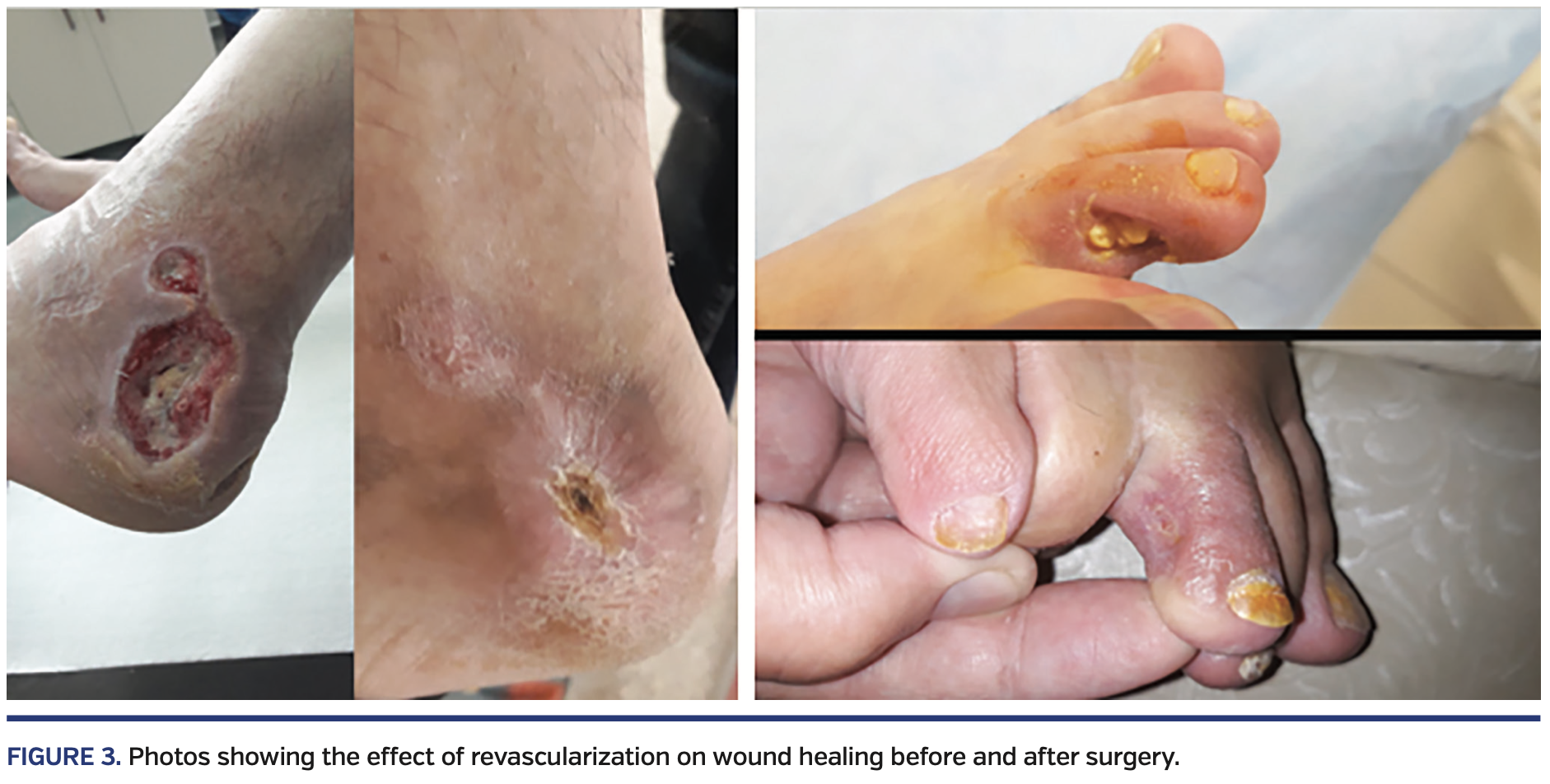
Six of the patients were Rutherford category 4, fifteen were category 5, and 3 were category 6. When the clinical situation was evaluated, the clinical response regarding improvement of 1 or more Rutherford categories was observed in 22 patients. As a result, the mean Rutherford category significantly improved from 5.2 ± 0.74 to 1.6 ± 0.7 (P<.001). ABI and SPP were examined in the clinical assessment. The mean ABI before the procedures was 0.47 ± 0.14 and increased to 1.06 ± 0.11 post intervention (P<.001). The SPP significantly increased from 28 ± 15 mm Hg to 61 ± 14 mm Hg post intervention (P<.001). Wound healing was achieved at 3.9 ± 2.6 months post revascularization (range, 1-13 months) (Figure 3).
Twenty-two patients were followed for 16.04 ± 9.07 months (range, 6-36 months). The patients’ ulcers healed and 6 early minor amputations were performed due to previously present necrotic tissues.
During follow-up, a total of 6 patients (2 with ischemic rest pain, 2 with ulcers, 2 with gangrene) required reintervention over a period of 3-21 months; 4 of these patients were smoking, and smoking was found to be strongly correlated with reintervention requirement (P<.01). The severity of ischemia was significantly less in non-smokers than in smokers (Rutherford category, 2.8 ± 1.4 vs 5.7 ± 0.6, respectively; P=.02). PTA was performed in 6 patients for 9 limbs (3 superficial femoral arteries, 3 popliteal arteries, 3 peroneal arteries, 1 tibioperoneal trunk, 7 posterior tibial arteries, and 6 anterior tibial arteries). Mortality or morbidity did not occur.
Periprocedural complications such as vessel perforations were observed in 3 patients. The perforations were at the superficial femoral artery in 2 patients and anterior tibial artery in 1 patient. Two perforations (1 superficial femoral artery and 1 anterior tibial artery) occurred during the primary intervention and the remaining perforation occurred during the secondary intervention. All perforations were immediately controlled by manual compression and/or balloon compression around the ruptured region. Desired interventions were then executed following control of the bleeding.
Discussion
Buerger’s disease is a challenging disease to treat and smoking cessation is the key therapeutic intervention. The literature lacks a completely satisfactory medical or surgical management for Buerger’s disease. The only common and definitive opinion about treatment is strict abstinence from tobacco. Medical therapy includes antiplatelets, calcium-channel blockers,10 and analgesics. Also, antibiotics are used in the management of superposed infections. Calcium-channel blockers resolve vasospasm and may increase the efficiency of oxygen use in the extremities.10
Bosentan, an oral endothelin antagonist, may be used for medical therapy of Buerger’s disease, as it is known that these patients have elevated endothelin serum levels. Although the drug is the subject of investigation in different studies, bosentan may improve clinical, angiographic, and endothelial functions.11,12 Injection of bone marrow and/or peripheral blood stem cells are considered as alternative therapeutic options in recent years and they have been shown to reduce major amputation rates in various studies.13,14
When patients with Buerger’s disease present with CLI, additional therapeutic interventions, including percutaneous or surgical revascularization measures, prostaglandin infusion, or sympathectomy, may be required.15 Since Buerger’s disease has a diffuse character and affects distal arteries, surgical revascularization is very challenging and is not appropriate for many patients. Patent distal vascular bed is mandatory for surgical revascularization; hence, in the case of severe ischemia, bypass with an autologous vein should be performed.16,17 In some studies, 5-year primary and secondary patency rates following infrainguinal bypass were found to be 49% and 62%, respectively.17 Additionally, the patency was higher in non-smokers at 67% and decreased to 35% in smokers. Omental transfer and in situ bypass techniques are other methods that may be attempted in patients with severe ischemia who have a distal vascular bed.18,19
Infusion of a prostaglandin, such as iloprost, is one treatment option for patients with Buerger’s disease.20 In some studies, prostaglandin analogues were found to be more efficient than lumbar sympathectomy.21 However, hospitalization requirement and side effects such as severe headache and facial flushing are disadvantages of prostaglandin infusion.20 Despite these disadvantages, iloprost is considered a beneficial and safe therapy that offers pain relief, ulcer healing, and decreased amputation rates.22
Sympathectomy is another treatment option for Buerger’s disease, and provides short-term pain relief and ulcer healing; however, long-term results are not as effective.23,24 At our institution, we observed that the beneficial effects of sympathectomy usually lasted less than 3 months. On the other hand, we did not hesitate to deliberately and recurrently use prostaglandin infusion in our patients.
Endovascular therapy for the treatment of Buerger’s disease is controversial due to the diffuse, inflammatory, and thrombotic condition of the disease. Technically, PTA in patients with Buerger’s disease is challenging.25 While prolonged balloon inflation is recommended for these patients, stenting is not recommended because of inflammation and tendency to clot.26 However, in cases of residual stenosis, dissection, or vascular recoil, stenting may need to be considered.26 In addition, the unpredictable response of the inflamed arteries in patients with Buerger’s disease has been one of the main reasons that many physicians refrained from percutaneous revascularization in this particular patient population.
Tibial artery recanalization may provide blood flow to the foot, and peroneal artery recanalization ensures collateral branches to the circulation of the foot.26 In recent years, a limited number of studies reported experiences on endovascular therapy for patients with Buerger’s disease. Graziani et al25 performed endovascular interventions on 20 limbs from 17 patients with technical success in 19 of 20 limbs (95%) and limb salvage at 100% over 23 months of follow-up. Clinical improvement was 84% and there was not a significant difference in limb salvage rates between smokers and non-smokers.25 A study by Kawarada et al27 was the first to show intravascular ultrasonography findings in patients with Buerger’s disease, with a mean follow-up of 26 months. Procedure success rate was 96% (complete and partial) and the patients had improvements of 1 or more Rutherford category grades.27 In another study, endovascular therapy was compared with autogenous venous bypass and was found to have comparable amputation-free survival, but lower primary and secondary patency rates.28 In addition, endovascular therapy has been associated with higher immediate failure and minor reintervention rates.28
Conclusion
Endovascular treatment of Buerger’s disease is challenging because of the unpredictable nature of the disease. Promising studies of endovascular interventions that report lower amputation rates, ulcer healing, and effective therapy in patients presenting to the clinic with CLI are encouraging.29 The influence of advances in medical technology on interventional therapy also cannot be overlooked. Hence, endovascular therapy may be considered as a first-line therapy, especially for limb salvage and wound healing in CLI patients as well as in patients with Buerger’s disease. In our modest cohort, although the revascularizations were determined based on the angiosome distributions of the wound or ulcer, we earnestly attempted to treat all feasible infrapopliteal arteries, which we believe further aided in promising outcomes in our series. In addition, the multidisciplinary wound team approach, psychological support, smoking rehabilitation, and deliberate use of medical agents aided in the promising outcomes in our patient population. These patients should be followed at short intervals, and smoking cessation and complete abstinence from nicotine products have utmost importance. Our results, along with many others, indicate that long-term durability correlates with the cessation of tobacco use. Multicenter and large-volume studies are warranted to identify the long-term efficiency of endovascular therapies in patients with Buerger’s disease.
Study limitations. The small number of patients and single-center nature of this study are the major limitations. In addition, this is a retrospective, non-comparative study, and does not include remote follow-up data.
From the 1Kadikoy Medicana Hospital, Cardiovascular Surgery Clinic, Istanbul, Turkey; 2Istanbul University Cerrahpasa, Institute of Cardiology, Department of Cardiovascular Surgery; 3Bagcilar Education and Research Hospital, Department of Cardiovascular Surgery, Istanbul, Turkey; 4Near East University, Department of Cardiology, Nicosia, Cyprus; 5Yedikule Chest Diseases and Thoracic Surgery Education and Research Hospital, Cardiovascular Surgery Clinic, Istanbul, Turkey; 6 Medipol University Medical Faculty, Department of Cardiovascular Surgery; 7Istanbul University Istanbul Medical Faculty, Department of Cardiovascular Surgery, Istanbul, Turkey; and 8Medical Sciences University, Umraniye Education and Research Hospital, Cardiovascular Surgery Clinic, Istanbul, Turkey.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted February 5, 2020, provisional acceptance given March 17, 2020, final version accepted April 9, 2020.
Address for correspondence: Dr Orhan Rodoplu, Kadikoy Medicana Hospital, Zuhtupasa Recep Peker Caddesi No:11, 34724 Mahallesi, 34724, Kadıkoy/Istanbul – Turkey. Email: orhanrodoplu01@yahoo.com
- Olin JW. Thromboangiitis obliterans. Curr Opin Rheumatol. 1994;6:44-49.
- Von Winiwarter F. Ueber eine eigenthümliche Form von Endarteriitis und Endophlebitis mit Gangrän des Fusses. Arch Klin Chir. 1879;23:202-226.
- Buerger L. Thrombo-angiitis obliterans: a study of the vascular lesions leading to presenile spontaneous gangrene. Am J Med. 1952;13:526-532.
- Olin JW. Thromboangiitis obliterans (Buerger’s disease). N Engl J Med. 2000;343:864-869.
- Modaghegh MS, Hafezi S. Endovascular treatment of thromboangiitis obliterans (Buerger’s disease). Vasc Endovascular Surg. 2018;52:124-130.
- Mills JL Sr. Buerger’s disease in the 21st century: diagnosis, clinical features, and therapy. Semin Vasc Surg. 2003;16:179-189.
- Shionoya S. Diagnostic criteria of Buerger’s disease. Int J Cardiol. 1998;66:S243-S245.
- Stoner MC, Calligaro KD, Chaer RA, et al. Reporting standards of the Society for Vascular Surgery for endovascular treatment of chronic lower extremity peripheral artery disease. J Vasc Surg. 2016;64:e1-e21.
- Martínez-De Jesús FR. A checklist system to score healing progress of diabetic foot ulcers. Int J Low Extrem Wounds. 2010;9:74-83.
- The European TAO Study Group. Oral iloprost in the treatment of thromboangiitis obliterans (Buerger’s disease): a double-blind, randomised, placebo-controlled trial. Eur J Vasc Endovasc Surg. 1998;15:300-307.
- Czarnacki M, Gacka M, Adamiec R. A role of endothelin 1 in the pathogenesis of thromboangiitis obliterans (initital news). Przegl Lek. 2004;61:1346-1350.
- De Haro J, Acin F, Bleda S, Varela C, Esparza L. Treatment of thromboangiitis obliterans (Buerger’s disease) with bosentan. BMC Cardiovasc Disord. 2012;12:5.
- Tateishi-Yuyama E, Matsubara H, Murohara T, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427-435.
- Moriya J, Minamino T, Tateno K, et al. Long-term outcome of therapeutic neovascularization using peripheral blood mononuclear cells for limb ischemia. Circ Cardiovasc Interv. 2009;2:245-254.
- Bagger J P, Helligsoe P, Randsbaek F, Kimose HH, Jensen BS. Effect of verapamil in intermittent claudication: a randomized, double-blind, placebo-controlled, cross-over study after individual dose-response assessment. Circulation. 1997;95:411-414.
- Sayin A, Bozkurt AK, Tuzun H, Vural FS, Erdog G, Ozer M. Surgical treatment of Buerger’s disease: experience with 216 patients. Cardiovasc Surg. 1993;1:377-380.
- Sasajima T, Kubo Y, Inaba M, Goh K, Azuma N. Role of infrainguinal bypass in Buerger’s disease: an eighteen-year experience. Eur J Vasc Endovasc Surg. 1997;13:186-192.
- Singh I, Ramteke VK. The role of omental transfer in Buerger’s disease: New Delhi’s experience. Aust N Z J Surg. 1996;66:372-376.
- Bozkurt AK, Beşirli K, Köksal C, et al. Surgical treatment of Buerger’s disease. Vascular. 2004;12:192-197.
- Fiessinger JN, Schafer M. Trial of iloprost versus aspirin treatment for critical limb ischaemia of thromboangiitis obliterans. The TAO study. Lancet. 1990;335:555-557.
- Bozkurt AK, Köksal C, Demirbas MY, et al. A randomized trial of intravenous iloprost (a stable prostacyclin analogue) versus lumbar sympathectomy in the management of Buerger’s disease. Int Angiol. 2006;25:162-168.
- Bozkurt AK, Cengiz K, Arslan C, et al. A stable prostacyclin analogue (iloprost) in the treatment of Buerger’s disease: a prospective analysis of 150 patients. Ann Thorac Cardiovasc Surg. 2013;19:120-125.
- Chander J, Singh L, Lal P, Jain A, Lal P, Ramteke VK. Retroperitoneoscopic lumbar sympathectomy for buerger’s disease: a novel technique. JSLS. 2004;8:291-296.
- Nesargikar PN, Ajit MK, Eyers P, Nichols BJ, Chester JF. Lumbar chemical sympathectomy in peripheral vascular disease: does it still have a role? Int J Surg. 2009;7:145-149.
- Graziani L, Morelli L, Parini F, et al. Clinical outcome after extended endovascular recanalization in Buerger’s disease in 20 consecutive cases. Ann Vasc Surg. 2012;26:387-395.
- Yuan L, Bao J, Zhao Z, Lu Q, Feng X, Jing Z. Clinical results of percutaneous transluminal angioplasty for thromboangiitis obliterans in arteries above the knee. Atherosclerosis. 2014;235:110-115.
- Kawarada O, Kume T, Ayabe S, et al. Endovascular therapy outcomes and intravascular ultrasound findings in thromboangiitis obliterans (Buerger’s disease). J Endovasc Ther. 2017;24:504-515.
- Ye K, Shi H, Qin J, et al. Outcomes of endovascular recanalization versus autogenous venous bypass for thromboangiitis obliterans patients with critical limb ischemia due to tibioperoneal arterial occlusion. J Vasc Surg. 2017;66:1133.e1-1142.e1.
- Samancı C, Önal Y. Percutaneous transluminal angioplasty of infrapopliteal arteries in patients with intermittent claudication: acute and six-month results. Haydarpasa Numune Med J. 2020;60:107-112.