Drug-Coated vs Uncoated Percutaneous Transluminal Angioplasty in Infrapopliteal Arteries: Six-Month Results of the Lutonix BTK Trial
Abstract: Objectives. We hypothesized that a drug-coated balloon (DCB) could improve treatment efficacy while maintaining safety when compared with percutaneous transluminal angioplasty (PTA) for the treatment of atherosclerotic infrapopliteal arterial lesions. Methods. A total of 442 patients with angiographically significant lesions were randomized (2:1) to DCB or PTA. The primary safety and efficacy endpoints were freedom from major adverse limb events and perioperative death (MALE-POD) at 30 days, and freedom from vessel occlusion, clinically driven target-lesion revascularization (CD-TLR), and above-ankle amputation measured at 6 months. Success was achieved if safety between groups was non-inferior (margin 12%), and efficacy was statistically significant either for the overall intention-to treat (ITT) or the proximal-segment DCB groups (ie, the proximal two-thirds of the below-knee arterial pathways). Results. Freedom from MALE-POD for the DCB group (99.3%) was non-inferior to PTA (99.4%; non-inferiority P<.001). Proportional analysis of the primary efficacy endpoint was statistically significant for the proximal-segment DCB group (76%) vs PTA (62.9%; one-sided P<.01; Bayesian P-value for success of .0085) while not statistically significant for the overall ITT group (74.5% for DCB vs 63.5% for PTA; one-sided P=.02). Kaplan-Meier analyses demonstrated superior efficacy for DCB in both the overall ITT and proximal-segment groups at 6 months. Primary patency and CD-TLR, hypothesis-tested secondary endpoints, were also statistically better for the DCB group compared with PTA at 6 months (one-sided P<.025). Conclusions. DCB treatment for symptomatic infrapopliteal arterial lesions produced non-inferior safety at 30 days and a statistically significant difference in the primary efficacy endpoint when compared with PTA at 6 months.
J INVASIVE CARDIOL 2019;31(8):205-211.
Key words: drug-coated balloon angioplasty, infrapopliteal disease, paclitaxel-coated balloon, percutaneous transluminal angioplasty, peripheral artery disease, peripheral vascular disease
Patients with advanced peripheral artery disease (PAD) and critical limb ischemia (CLI) face an elevated risk of amputation and a mortality rate higher than most cancers.1,2 Surgical and catheter-based revascularization are recommended to restore distal perfusion and prevent amputation in symptomatic patients.3,4 Surgical bypass is effective when experienced surgeons use a suitable autogenous vein, and when the anatomic and patient risk factors permit this approach.5 Patients with CLI are often poor surgical candidates because of the absence of suitable venous conduit or the presence of significant medical comorbidities.6 Percutaneous transluminal angioplasty (PTA) is the standard endovascular treatment for hemodynamically significant infrapopliteal lesions.7,8 Although initial technical success using PTA often exceeds 90%, restenosis necessitating repeat revascularization or amputation is common.7,8 Percutaneous treatment with balloons or stents that deliver antiproliferative agents directly to the vessel wall may inhibit neointimal hyperplasia and lower the risk of restenosis.9-11 To date, however, randomized trials in infrapopliteal arteries have provided mixed results.12-14 In the Levant II study, the paclitaxel-based drug-coated balloon (DCB) used in the current trial demonstrated superior patency compared with standard, uncoated PTA in patients with femoropopliteal artery disease.15 The purpose of the current trial was to evaluate the safety and efficacy of the same DCB in patients with infrapopliteal PAD.
Methods
Study design and oversight. The prospective, multicenter, randomized, single-blind, concurrently controlled Lutonix below-the-knee (Lutonix BTK) study compared the use of a paclitaxel DCB to uncoated PTA in the treatment of obstructive lesions in the distal popliteal, anterior tibial, posterior tibial, and peroneal arteries. The trial protocol was approved by the institutional review board or ethics committee at each investigative site, as well as the Center for Devices and Radiologic Health (CDRH) of the Food and Drug Administration (FDA). Patients were advised of the risks and potential benefits of treatment and provided written informed consent prior to participation. Procedures were conducted in accordance with the Declaration of Helsinki, good clinical practices, and other applicable healthcare regulations in the United States, Canada, Europe, and Japan. Data were collected by on-site investigators on electronic case report forms and monitored by either clinical research associates employed by the sponsor or by contract research organizations paid by the sponsor. Data monitoring and clinical events committees provided independent oversight of patient safety. The study was sponsored by Lutonix in support of an investigational device exemption and registered on clinicaltrials.gov (NCT01870401) prior to patient enrollment.
Study population and procedures. Clinical and angiographic inclusion and exclusion criteria are listed in Supplemental Table S1. Eligible patients initially had symptoms of CLI (Rutherford categories 4 and 5) while patients with severe claudication (Rutherford category 3) were added later in the study by protocol amendment.16 After meeting clinical eligibility criteria, patients received an angiographic examination to confirm that the lesion could be treated with PTA and that the atherosclerotic stenosis was at least 70%. As specified by the study protocol, a patent inflow artery from the aorta to the target lesion was confirmed by angiography; treatment of inflow arteries (ie, iliac, superficial femoral, or above-knee popliteal) was allowed if successfully treated without major vascular complication. DCB treatment of inflow arteries was prohibited. Multiple lesions in up to two native infrapopliteal arterial pathways, between the tibial plateau and tibiotalar joint, could be treated; the total treated length could not exceed 320 mm, the reference vessel diameter had to be between 2 and 4 mm, and the target lesion had to be at least 20 mm from any previously deployed stent.
![SUPPLEMENTAL FIGURE S1. Patient disposition through 6 months. A total of 462 patients were enrolled at 51 sites in the Unit - ed States, Europe, Japan, and Canada — 10 were roll-in training cases that were treated with the drug-coated balloon (DCB) and followed as a separate cohort; 10 patients did not fit the criteria for randomization after initial predilation, were treated according to standard of care, and were screened for safety at 30 days (standard-practice subgroup); and the remaining 442 patients were randomized (2:1) to either DCB (n = 287) or percutaneous transluminal angioplasty (PTA) (n = 155). Overall, 83.7% of the inten - tion-to-treat population completed a 6-month evaluation (370/442 patients). Thirty-three patients discontinued participation in the study prior to 6 months (22 [7.7%] in the DCB group and 11 [7.1%] in the PTA group). An additional 39 patients did not complete a 6-month evaluation, but are still enrolled in the study and potentially available for longer-term follow-up examination](https://d148x66490prkv.cloudfront.net/hmp_ln/inline-images/Table%20S1_5.png)
After meeting angiographic eligibility criteria, patients underwent predilation with a standard angioplasty balloon. Multiple balloons and inflations, as well as prolonged inflations, were allowed, but specialty balloons (eg, cutting or scoring balloons) were not permitted. Following predilation, the patient was considered enrolled in the study. Patients with a postangioplasty residual stenosis >50% were excluded from randomization, treated according to the investigator’s standard of care, and followed for 30 days for safety outcomes. Patients meeting study criteria after predilation were stratified by Rutherford category and randomly assigned in a 2:1 fashion to undergo infrapopliteal angioplasty with a DCB (Lutonix; Bard Peripheral Vascular) or PTA. The Lutonix DCB was coated with a 2 µg/mm2 dose of paclitaxel, an excipient of polysorbate and sorbitol, and supplied on a 0.014˝-compatible over-the-wire catheter, while PTA was performed with any commercially available balloon chosen at the discretion of the investigator. Provisional bare-metal stent placement was allowed, if necessary, as a bailout procedure in cases of flow-limiting dissection or recoil. Patients, core laboratory evaluators, and members of the clinical events committee (CEC) were blinded to the treatment received. Investigators and their clinical teams, however, could not be blinded due to the visible differences in appearance between the DCB and standard angioplasty balloons.
An anticoagulation regimen was suggested as part of the study plan; recommended therapies varied by geographic region, however, and specifics were left to local hospital practice. Starting 3 days before the procedure, acetylsalicylic acid was recommended at a dose of 75-325 mg/day, along with a loading dose of clopidogrel (300 mg), ticagrelor (180 mg), or prasugrel (60 mg). Following the procedure, dual-antiplatelet therapy with acetylsalicylic acid (75-100 mg/day) and clopidogrel (75 mg/day), ticagrelor (180 mg/day), or prasugrel (5-10 mg/day, depending on body weight) was recommended for at least 1 month, and a dose of 75-100 mg of acetylsalicylic acid was suggested indefinitely thereafter.
Angiography was required during the procedure, and at the time of any reintervention. Duplex ultrasonography (DUS) was required after completion of the procedure, at all follow-up visits, and at the time of any reintervention. In addition, clinical evaluations (consisting of physical examination, wound assessment, and adverse events), assessment of limb hemodynamics, and a health-related quality-of-life (QoL) questionnaire were completed at all follow-up visits. Treatment decisions (in particular the need for reintervention) were based on patient symptoms at follow-up. Accordingly, patients completed QoL evaluations prior to follow-up physical examinations, and investigators completed clinical evaluations and indicated if reintervention was clinically necessary prior to viewing DUS exam results. SynvaCor, the Core Laboratory at Prairie Education and Research Cooperative (Springfield, Illinois) independently analyzed angiographic images while VasCore, the Vascular Ultrasound Core Laboratory at Massachusetts General Hospital (Boston, Massachusetts) independently reviewed DUS images.
Study endpoints. The primary safety measure was a composite of freedom from major adverse limb events and perioperative death (MALE-POD) at 30 days adjudicated by the CEC; a major adverse limb event was defined as above-ankle amputation or major reintervention (ie, new bypass graft, interposition graft revision, or thrombectomy/thrombolysis) of the treated limb involving a BTK artery. The primary efficacy endpoint was a composite of primary patency and freedom from above-ankle amputation measured at 6 months. Primary patency was defined as freedom from vessel occlusion (<100% stenosis), determined by the angiographic or DUS core laboratories, as well as freedom from clinically driven target-lesion revascularization (CD-TLR, defined as reintervention due to delayed or worsening wound healing, new or recurrent wound, or worsening Rutherford class) adjudicated by the CEC.
Patients with severe PAD and CLI have poor vessel compliance because of the concentration of calcium in the arterial wall.17-19 This lack of compliance is most prevalent in the more distal infrapopliteal arteries, increasing the risk of acute vessel recoil following PTA and leading to restenosis. Since recoil negatively impacts clinical outcomes following angioplasty, the primary efficacy endpoint in the current trial was calculated for both all arterial flow pathways in the intention-to-treat (ITT) population as well as for the proximal-segment flow pathways. The proximal segment was calculated by the angiographic core laboratory based on the total length of the BTK arterial segment measured from the tibial plateau to the ankle; the proximal two-thirds of this overall length was considered the proximal segment flow pathways.
Secondary efficacy measures included: CD-TLR; primary patency; change in patient QoL as measured by the Euro-QoL Group 5-Dimension (EQ-5D) Self-Reporting Questionnaire (scores ranged from 1 to 5, with lower scores indicating a better quality of life); change in Rutherford class; Walking Impairment Questionnaire (WIQ) scores (scores ranged from 0 to 100, with lower scores indicating more difficulty in walking); and hemodynamic outcome, a measurement of the change in toe and ankle pressures of the treated limb. Secondary safety measures included: wound healing, an observational status of patients with wounds at baseline compared to follow-up (scored as improved, stagnant, or worse); freedom from major amputation (above-ankle); and all-cause death.
Statistical analysis. The maximum sample size of 840 treated vessels (allocated in a ratio of 2 DCB to 1 PTA) was calculated to provide 93% power to detect a 6-month primary efficacy endpoint difference of 15% between groups at a one-sided alpha of 0.025 (assumption: 55% in the DCB group and 40% in the PTA group). It was also adjusted for a 15% patient attrition rate to account for study withdrawal or missing imaging data. A Bayesian adaptive design incorporated interim analyses to determine the final sample size for the study. A minimum sample size of 300 and a maximum of 840 were set with the final estimate to be based on the predictive probability of primary-efficacy success. The adaptive design included planned interim analyses after 400, 500, 600, and 700 vessels were treated to calculate the predictive probability of trial success. Enrollment was stopped, however, for administrative reasons after 507 vessels were treated; patient accrual was low and slowing at that point, and the study sponsor felt clinical-benefit questions could be addressed with the available data.
Primary efficacy and safety analyses were performed on an ITT basis, with patients analyzed as randomized regardless of treatment received through the close of the 6-month follow-up window. The primary efficacy endpoint was analyzed per arterial flow pathway using logistic regression to account for the possibility of multivessel treatment in some patients; it was calculated for all flow pathways and for the proximal-segment flow pathways (ie, proximal two-thirds of the BTK flow pathway measured by the angiographic core laboratory). To preserve the type-I error level for the primary efficacy analysis below 0.025, the P-value was set at .0085 (one-sided test). Success of one or both efficacy analyses was considered success of the primary efficacy endpoint. The primary safety endpoint was analyzed per patient using a Farrington-Manning test for non-inferiority of proportions with a non-inferiority margin of 12% and a one-sided P-value of .025. Kaplan-Meier (K-M) estimates were included in addition to proportional analyses, where applicable, to account for missing data (eg, death, un-interpretable imaging data, or withdrawal from the study); survival estimates were presented with two-sided 95% confidence intervals (CIs) and log-rank P-values (one-sided P-value for success of .025). Success of the primary efficacy and safety endpoints triggered sequential hypothesis testing of four secondary outcomes – primary patency excluding early (ie, ≤30 days) mechanical recoil, primary patency (ie, freedom from total occlusion and CD-TLR), freedom from CD-TLR, and one secondary composite safety endpoint (ie, freedom from amputation, unhealed wound, resting pain, target-vessel occlusion, and clinically driven TVR). Descriptive statistics included frequency counts and percentages along with 95% CIs. Summary statistics, including mean, standard deviation, and 95% CIs were provided for continuous variables. Statistical analyses were performed using SAS version 9.4 (SAS Institute).
Results
Study population and treatment. Between June 2013 and December 2017, a total of 462 patients were enrolled at 51 investigative centers in the United States, Europe, Japan, and Canada. Supplemental Figure S1 details enrollment and distribution of patients. Of the total, ten were roll-in training cases that were treated with the DCB and followed as a separate cohort. Ten patients did not fit the criteria for randomization after initial predilation, were treated according to standard of care, and were screened for safety at 30 days (standard-practice subgroup). The remaining 442 patients were randomly assigned (2:1) to either treatment with DCB (287 patients) or standard PTA (155 patients). Of the overall ITT population, 420 patients (95%) were considered by the angiographic core laboratory to have lesions in the proximal BTK flow segment.
![SUPPLEMENTAL FIGURE S1. Patient disposition through 6 months. A total of 462 patients were enrolled at 51 sites in the Unit - ed States, Europe, Japan, and Canada — 10 were roll-in training cases that were treated with the drug-coated balloon (DCB) and followed as a separate cohort; 10 patients did not fit the criteria for randomization after initial predilation, were treated according to standard of care, and were screened for safety at 30 days (standard-practice subgroup); and the remaining 442 patients were randomized (2:1) to either DCB (n = 287) or percutaneous transluminal angioplasty (PTA) (n = 155). Overall, 83.7% of the inten - tion-to-treat population completed a 6-month evaluation (370/442 patients). Thirty-three patients discontinued participation in the study prior to 6 months (22 [7.7%] in the DCB group and 11 [7.1%] in the PTA group). An additional 39 patients did not complete a 6-month evaluation, but are still enrolled in the study and potentially available for longer-term follow-up examination](https://d148x66490prkv.cloudfront.net/hmp_ln/inline-images/Figure%20S1_1.png)
Baseline patient demographic and medical histories are summarized in Supplemental Table S2. Patients were well matched, with both groups exhibiting medical histories and risk factors expected of patients with PAD. Mean patient age was 73 years, most (69%) were male, and 56.1% in both groups presented with ischemic tissue loss (Rutherford category 5). The majority of patients were hypertensive (93.2% in the total population), diabetic (70.1%), had high cholesterol (77.1%), were current or former smokers (58.6%), and had undergone previous peripheral vascular interventions (53.8%). Baseline lesion characteristics are provided in Supplemental Table S3. A total of 605 lesions were treated in 507 flow pathways (ie, one or more contiguous arterial segments that provided in-line flow to the foot following treatment) in the ITT population (380 lesions in 323 flow pathways in the DCB group; 225 lesions in 184 flow pathways in the PTA group); 476 of the arterial flow pathways were located in the proximal segment (304 in the DCB group; 172 in the PTA group). The most common lesion location was the anterior tibial artery (38.4% in the DCB group; 36% in the PTA group), followed by the tibial-peroneal trunk (23.9% vs 25.3%, respectively), the posterior tibial artery (23.7% vs 25.8%, respectively), and the peroneal artery (23.4% vs 20.9%, respectively). The mean total lesion length, measured by the angiographic core laboratory, was 111.8 ± 92.6 mm in the DCB group and 94.7 ± 85.4 mm in the PTA group. Total occlusions accounted for 36.1% of lesions in the DCB group and 33.3% of lesions in the PTA group, lesions were reported as calcified in 59.9% of the DCB group vs 54.2% of the PTA group, and TASC C and D lesions were reported in 30.8% of the DCB group vs 22.0% of the PTA group.

Absence of an angiographically confirmed inflow obstruction (≥50%) was required for study enrollment; therefore, treatment of inflow vessels was allowed prior to or during the index procedure. Inflow lesions were treated in 34.8% of DCB patients and 28.4% of PTA patients. The most common inflow location treated was the superficial femoral artery (71.4% of the DCB group and 72.7% of the PTA group), while the most common inflow treatment procedure was a combination of PTA and provisional stent placement, observed in 41.3% of pathways in the DCB group and 41.8% of pathways in the PTA group. No major vascular complications were reported during inflow treatment. Vascular access was achieved through the femoral artery in 99.5% of cases, with an additional ipsilateral, retrograde access obtained in 7.7% of the DCB cases and 10.3% of the PTA cases (Supplemental Table S3). A single BTK flow pathway was treated in most cases (87.8% of the DCB group and 76.8% of the PTA group), although treatment of lesions in up to two parallel flow pathways was allowed. Angiographic analysis after completion of the procedure demonstrated a final mean residual stenosis of 29.5 ± 13.8% in the DCB group and 30.0 ± 12.8% in the PTA group.


Postprocedure follow-up and endpoint analyses. Six-month data were evaluated for this analysis. Overall, 83.7% of the ITT population completed a 6-month evaluation (370/442 patients). Thirty-three patients discontinued participation in the study prior to 6 months; 22 (7.7%) in the DCB group and 11 (7.1%) in the PTA group. Fourteen patients in the DCB group and 6 patients in the PTA group died prior to their 6-month follow-up, 6 patients in the DCB group and 3 patients in the PTA group withdrew consent to participate, and 4 patients were either lost to follow-up or were removed for other reasons (2 DCB patients and 2 PTA patients). An additional 39 patients did not complete a 6-month evaluation (20 DCB patients and 19 PTA patients), are still enrolled in the study, and are potentially available for longer-term follow-up examination (Supplemental Figure S1).

Primary endpoint analyses are summarized in Supplemental Table S4. The primary safety endpoint, freedom from 30-day MALE-POD for the DCB group (99.3%) was statistically non-inferior to the PTA group (99.4%; P<.001 with a non-inferiority margin of 12%). Safety data were available for 440 patients and were evaluated by the CEC. The composite primary efficacy measure – freedom from vessel occlusion, CD-TLR, and above-ankle amputation – was measured per flow pathway rather than per patient, and was calculated for both the proximal-segment group and the overall ITT population; vessel occlusion was confirmed by the angiographic or DUS core laboratories, and CD-TLR was adjudicated by the CEC. At 6 months, a total of 386 flow pathways (81.1%) were available for binary, proportional analysis in the proximal-segment analysis group. The composite success rate was 76.0% (193/254; 95% CI, 70.2-81.1) for the DCB group and 62.9% (83/132; 95% CI, 54.0-71.1) for the PTA group; the mean difference of 13.1% between groups was statistically significant (one-sided P=.0079, compared to the Bayesian one-sided P-value for success of .0085). When evaluated for the 404 flow pathways (79.7%) in the overall ITT population where data were available for analysis at 6 months, the rate of primary efficacy success was 74.5% (199/267; 95% CI, 68.9-79.6) for the DCB group vs 63.5% (87/137; 95% CI, 54.9-71.6) for the PTA group; the mean rates were numerically different (11.0%), but not statistically significant (one-sided P=.0179, compared to the Bayesian one-sided P-value for success of .0085). To account for missing data in the proportional analyses, freedom from primary efficacy failure (i.e., freedom from occlusion, CD-TLR, or above-ankle amputation) was also evaluated by Kaplan-Meier analysis (Figure 1); there was a statistically significant difference in the means between groups favoring treatment with DCB for both the proximal-segment and the overall populations at 180 days (P<.001). Freedom from primary efficacy failure for the proximal-segment population was 86.2% for the DCB group and 69.9% for the PTA group, a mean difference of 16.3% (Figure 1A), while the mean difference between the DCB group (85.8%) and the PTA group (70.7%) for the overall ITT population was 15.1% (Figure 1B).

Based on successfully meeting the composite primary safety and efficacy endpoints, four protocol-prescribed secondary endpoints were hypothesis tested in sequence at 6 months (Supplementary Table S4). Three secondary efficacy endpoints were evaluated for the proximal segment – primary patency excluding early (≤30 days) mechanical recoil, primary patency, and freedom from CD-TLR – while one secondary safety endpoint was evaluated for the overall ITT population – freedom from amputation, unhealed wound, resting pain, target-vessel occlusion, and clinically driven TVR. The DCB group performed statistically better than the PTA group for the three secondary efficacy parameters. Primary patency at 6 months, excluding early mechanical recoil, was 77.8% for the DCB group vs 65.6% for the PTA group (P=.01), freedom from total occlusion and CD-TLR (ie, primary patency) at 6 months was 76.9% for the DCB group vs 64.3% for the PTA group (P=.01), and freedom from CD-TLR was 91.3% for the DCB group vs 81.4% for the PTA group (P<.01). The hypothesis-tested secondary safety endpoint was not statistically different between the two treatment groups at 6 months (P=.41).
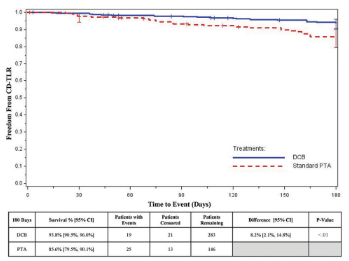
Survival curves (K-M) for CD-TLR and primary patency for the overall ITT analysis population are displayed in Supplemental Figures S2 and S3. Freedom from CD-TLR was 93.8% (95% CI, 90.5-96.0) for the DCB group vs 85.6% (95% CI, 79.3-90.1) for the PTA group, a mean difference of 8.2% (95% CI, 2.1-14.8) at 180 days (P<.01). Primary patency, the absence of occlusion and CD-TLR, was 86.7% (95% CI, 82.1-90.2) for the DCB group vs 72.2% (95% CI, 64.0-78.9) for the PTA group – a mean difference of 14.5% (95% CI, 5.4-23.5) at 180 days (P<.001). Patency was analyzed by the angiographic or DUS core laboratory, and CD-TLR was adjudicated by the CEC.

Additional secondary outcomes for the overall ITT population are summarized in Supplemental Table S5. Numerical differences were observed between the DCB and PTA groups, but the differences in secondary outcomes did not reach statistical significance. The mean index score at 6 months with the EQ-5D was 0.74 ± 0.24 in the DCB group and 0.73 ± 0.28 in the PTA group, with similar improvement from baseline (0.07 ± 0.3 and 0.05 ± 0.3, respectively). The mean improvement in Rutherford categories from baseline to 6 months was -2.5 ± 2.0 categories for the DCB group vs -3.0 ± 1.8 for the PTA group; mean improvement in the WIQ total score was 2.9 ± 21.6 for the DCB group vs 3.6 ± 20.3 for the PTA group; the mean change in ankle-brachial index (ABI) was 0.16 ± 0.36 for the DCB group vs 0.17 ± 0.43 for the PTA group; and the mean change in toe-brachial index (TBI) was 0.15 ± 0.24 for the DCB group vs 0.09 ± 0.29 for the PTA group. Wound status was qualitatively evaluated by the investigative site at each follow-up visit; if a wound that was present at baseline had not healed, the site reported the status as improved, stagnant, or worse. The number of patients with wounds at baseline was similar in both treatment groups (59.0% in the DCB group vs 58.0% in the PTA group). At 6 months, wounds were reported as not healed in 30.7% of the DCB group vs 21.0% of the PTA group; of the non-healed wounds, 51.0% of the DCB group vs 35.3% of the PTA group were reported as improving. Infected wounds were reported in 25.9% of DCB patients vs 26.5% of PTA patients at baseline, while infected wounds were observed in 5.5% of the DCB group and 16.3% of the PTA group at 6 months. Gangrene was reported in 22.0% of the DCB group and 21.2% of the PTA group at baseline, while gangrenous wounds were observed in 7.4% of DCB patients and 6.3% of PTA patients at 6 months. Freedom from above-ankle amputation at 6 months was 98.9% in the DCB group vs 98.0% in the PTA group (K-M survival estimate).

There were 14 deaths (5.0%) in the DCB group and 6 deaths (4.0%) in the PTA group through 6 months. Deaths in the DCB group were due to respiratory insufficiency and failure (n = 4), metastatic bladder cancer, pancreatic cancer, heart failure and cardiac arrest (n = 8), non-treatment limb gangrene (the patient refused treatment), and unknown cause (n = 1). The causes of death in the PTA group were pneumonia, congestive obstructive pulmonary disease, respiratory failure, acute heart failure, congestive heart failure, and clostridium with uncontrolled diarrhea. All deaths through 6 months were adjudicated by the CEC, and no deaths were determined to be related to the device or procedure. Freedom from all-cause death at 180 days (K-M survival estimate) was 96.8% (95% CI, 93.9-98.3) for the DCB group and 96.0% (95% CI, 91.4-98.2) for the standard PTA group (no difference between groups at 6 months; observational one-sided P=.70).
Discussion
We compared drug-coated to uncoated PTA for the treatment of obstructive, atherosclerotic lesions in the BTK arteries (ie, popliteal, tibial, and peroneal arteries), and found that safety, a composite of freedom from MALE-POD at 30 days, for the Lutonix DCB group was non-inferior to PTA (99.3% vs 99.4%, respectively) while efficacy, defined as freedom from above-ankle amputation, occlusion, and CD-TLR, was statistically better for the proximal segment DCB group vs the PTA group (a difference of 13.1%; one-sided P=.0079) at 6 months. Primary patency and CD-TLR, hypothesis-tested secondary endpoints, also demonstrated statistically significant differences in outcomes favoring the DCB group at 6 months (one-sided P-values <.025).
Patients with CLI from infrapopliteal atherosclerotic disease comprise a challenging population, most often accompanied by multilevel arterial disease with limb-threatening pathology and comorbidities that account for a high mortality rate.8 Previous trials of interventional therapies to treat infrapopliteal disease have provided mixed results. Romiti et al systematically reviewed 30 studies and provided a meta-analysis of PTA for the treatment of infrapopliteal arterial disease; they concluded that the long-term durability of angioplasty alone was lower than surgical bypass, but PTA provided acceptable rates of limb salvage and overall survival that were equivalent to bypass surgery.20 Three randomized trials (YUKON-BTK, DESTINY, and ACHILLES), evaluating over 500 patients, compared the use of coronary drug-eluting stents (sirolimus- or everolimus-eluting) to PTA or bare-metal stents.21-23 Katsanos et al pooled the results from these studies and reported a primary patency rate of 80% for the drug-coated stents vs 58.5% for the PTA and bare-metal stent controls at 1 year.24 Similarly, the rates of TLR, event-free survival, and wound healing were better for the drug-eluting stent group; however, the lesions treated were focal, ranging in length from 16-31 mm, and were not representative of the typical clinical patient presenting with lower-limb disease and CLI. Maximum lesion lengths in the current trial were much longer, ranging from 340-361 mm (mean total lesion length of 111.8 mm in the DCB group), with primary efficacy in the longest-lesion quartile (182-360 mm) of 65.7% for the DCB group vs 32.3% for the PTA group using logistic regression covariate analysis. An early, single-center randomized trial of 132 patients with 158 infrapopliteal arterial lesions treated with DCB (DEBATE-BTK) demonstrated that binary restenosis was 27% in patients treated with DCB vs 74% in the PTA group (P<.001), the rate of TLR was lower in the DCB group, and wounds healed in 86% of the patients in the DCB group vs 67% in the PTA group at 12 months.25 A multicenter, randomized study of 358 patients randomized to DCB vs PTA found that CD-TLR was 9.2% in the DCB group vs 13.1% in the PTA group (P=.29), but there was a numerically higher rate of amputation at 12 months (8.8% in the DCB group vs 3.6% in the PTA group; P=.08).12
Early findings from the Lutonix BTK study are encouraging, but longer-term follow-up is needed to determine the clinical benefit of DCB use for obstructive infrapopliteal arterial lesions. The trial at 6 months met the primary safety and efficacy endpoints of non-inferior MALE-POD between groups and a statistically significant difference favoring DCB for primary efficacy in the proximal BTK segment. Over 50% of wounds were reported as improving in the DCB group vs 35% in the PTA group, and fewer infected wounds were reported in the DCB group at 6 months (5.6% vs 16.3%, respectively). Other secondary observations, however, were similar between the two groups at 6 months (eg, improvement in Rutherford scores, quality of life measures, and hemodynamic improvements). No deaths were adjudicated as related to the devices or the procedure, and the rates of all-cause death were similar between groups at 6 months (4.9% in the DCB group vs 3.9% in the PTA group). The trial protocol mandates clinical follow-up through 3 years, and that data will augment the early results presented herein.
In addition to short-term follow-up, there were other limitations to the trial. Patients, core laboratory staff, and members of the CEC were blinded to the treatment received; however, the investigators performing the study procedures were aware of the specific devices used, thereby introducing potential investigator bias. Outcomes may not be specific to just the study lesions and treatment received; patients with CLI have severe, progressive PAD with multiple comorbidities and high rates of mortality and limb loss. The two-part efficacy endpoint (ie, overall ITT population or the proximal segment group) and the Bayesian adaptive design provided a strict threshold for efficacy success (P-value of .0085). Proportional analysis of the composite efficacy endpoint for the overall ITT population did not reach this strict level of statistical significance; however, when estimated by K-M analysis, to account for patients missing from the proportional analysis (eg, those who died, had un-interpretable imaging data, or withdrew from the study), the difference between the DCB group and PTA group in the overall ITT population reached statistical significance (one-sided P<.001).The use of DCB or standard PTA does not cure systemic atherosclerotic disease, so changes in outcomes may be due in part to the progression of disease rather than to a difference in the devices used. Predilation was performed by standard angioplasty alone; the effects of vessel preparation using atherectomy or cutting balloons or focal-force balloons to debulk the treatment area or score the lesions are unknown.
Conclusion
In patients with symptomatic infrapopliteal PAD, treatment with a paclitaxel drug-coated angioplasty balloon provided non-inferior safety to uncoated PTA at 30 days and a statistically significant difference in composite efficacy favoring treatment with DCB at 6 months. Primary patency and CD-TLR, both hypothesis-tested secondary endpoints, also demonstrated statistically significant differences in outcomes favoring the DCB group at 6 months. Clinical and functional outcomes were similar between groups at this early timepoint, with more complete analysis and longer-term follow-up needed to determine whether DCB provides significant long-term clinical benefit to patients with severe, infrapopliteal arterial disease.
Acknowledgment. The authors wish to thank Joseph Giorgianni, Anna Lovas, and Lisa Erickson for their management of the clinical trial and assistance with clinical data.
From the 1Advanced Cardiac and Vascular Centers for Amputation Prevention, Grand Rapids, Michigan; 2Division of Angiology, Medical University Graz, Graz, Austria; 3Department of Surgery, Washington University School of Medicine, St. Louis, Missouri; 4Medical Affairs, Beckon, Dickinson and Company, Tempe, Arizona; and 5Newton-Wellesley Hospital, Newton, Massachusetts.
Funding: The study was sponsored in support of an Investigation Device Exemption by Lutonix, Inc, a wholly-owned subsidiary of Becton, Dickinson and Company.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Brodmann reports no conflicts of interest regarding the content herein. Dr Geraghty reports personal fees from Bard Peripheral Vascular/Lutonix, Boston Scientific, Intact Vascular, and Zimmer Biomet; equity ownership in Euphrates Vascular/Pulse Therapeutics. Dr Jaff reports non-financial support from Biotronik, Medtronic, Abbott Vascular, and Boston Scientific; personal fees from AOPA, Micell, Primacea, Sanofi, Vactronix, and Volcano/Philips; equity in Embolitech, Gemini, PQ Bypass, Primacea, Sano V, and Vascular Therapies. Dr Mustapha reports personal fees from Cardiovascular Systems, Inc, Bard Peripheral Vascular, Micromedical Solutions (Chief Medical Officer), Philips, Reflow Medical, and Terumo Medical; grants and personal fees from Boston Scientific and PQ Bypass; grant funds, board of directors, and equity in CardioFlow. Dr Saab reports personal fees from Bard Peripheral Vascular, Cardiovascular Systems, Inc, Philips, Terumo Medical, grants and personal fees from Boston Scientific; grants from CardioFlow and PQ Bypass. Mr Settlage is an employee of Becton, Dickinson and Company, the study sponsor.
Manuscript submitted May 20, 2019, accepted May 30, 2019.
Address for correspondence: Jihad A. Mustapha, MD, Advanced Cardiac and Vascular Centers for Amputation Prevention, 1525 E. Beltline, NE, Suite 101, Grand Rapids, MI 49525. Email: jmustapha@acvcenters.com; Twitter: @Mustapja
References
1. Mustapha JA, Katzen BT, Neville RF, et al. Disease burden and clinical outcomes following initial diagnosis of critical limb ischemia in the Medicare population. JACC Cardiovasc Interv. 2018;11:1011-1012.
2. Farber A. Chronic limb-threatening ischemia. N Engl J Med. 2018;379:171-180.
3. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;69:1465-1508.
4. Aboyans V, Ricco, J-B, Batelink, M-LEL, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2018;55:305-368.
5. Bradbury AW. Bypass versus angioplasty in severe ischemia of the leag (BASIL) trial in perspective. J Vasc Surg. 2010;51:IS-4S.
6. Conte MS. Critical appraisal of surgical revascularization for critical limb ischemia. J Vasc Surg. 2013;57:8S-13S.
7. Razavi MK, Mustapha JA, Miller LE. Contemporary systematic review and meta-analysis of early outcomes with percutaneous treatment for infrapopliteal atherosclerotic disease. J Vasc Interv Radiol. 2014;25:1489-1496.
8. Mustapha JA, Finton SM, Diaz-Sandoval LJ, Saab FA, Miller LE. Percutaneous transluminal angioplasty in patients with infrapopliteal arterial disease: systematic review and meta-analysis. Circ Cardiovasc Interv. 2016;9:e003468.
9. Scheller B, Speck U, Schmitt A, Bohm M, Nickenig G. Addition of paclitaxel to contrast media prevents restenosis after coronary stent implantation. J Am Coll Cardiol. 2003;42:1415-1420.
10. Scheller B, Speck U, Abramjuk C, Bernhardt U, Bohm M, Nickenig G. Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circulation. 2004;110:810-814.
11. Dake MD, Ansel GM, Jaff MR, et al. Durable clinical effectiveness with paclitaxel-eluting stents in the femoropopliteal artery: 5-year results of the Zilver PTX trial. Circulation. 2016;133:1472-1483.
12. Zeller T, Baumgartner I, Scheinert D, et al. Drug-eluting balloon versus standard balloon angioplasty for infrapopliteal arterial revascularization in critical limb ischemia: 12-month results from the IN.PACT DEEP randomized trial. J Am Coll Cardiol. 2014;64:1568-1576.
13. Siablis D, Kitrou PM, Spiliopoulos S, Katsanos K, Karnabatidis D. Paclitaxel-coated balloon angioplasty versus drug-eluting stenting for the treatment of infrapopliteal long-segment arterial occlusive disease: the IDEAS randomized controlled trial. JACC Cardiovasc Interv. 2014;7:1048-1056.
14. Fanelli F, Cannavale A, Corona M, Lucatelli P, Wlderk A, Salvatori FM. The “DEBELLUM” — lower limb multilevel treatment with drug eluting balloon — randomized trial: 1-year results. J Cardiovasc Surg (Torino). 2014;55:207-216.
15. Rosenfield K, Jaff MR, White CJ, et al. Trial of a Paclitaxel-Coated Balloon for Femoropopliteal Artery Disease. N Engl J Med. 2015;373:145-153.
16. Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517-538.
17. Baumann F, Fust J, Engelberger RP, et al. Early recoil after balloon angioplasty of tibial artery obstructions in patients with critical limb ischemia. J Endovasc Ther. 2014;21:44-51.
18. Mustapha JA, Diaz-Sandoval L, Saab F. Infrapopliteal calcification patterns in critical limb ischemia: diagnostic, pathologic, and therapeutic implications in the search for the endovascular holy grail. J Cardiovasc Surg. 2017;58:383-401.
19. Fanelli F, Cannavale A, Gazzetti P, et al. Calcium burden assessment and impact on drug-eluting balloons in peripheral arterial disease. Cardiovasc Intervent Radiol. 2014;37:898-907.
20. Romiti M, Albers M, Brochado-Neto FC, Durazzo AE, Pereira CA, De Luccia N. Meta-analysis of infrapopliteal angioplasty for chronic critical limb ischemia. J Vasc Surg. 2008;47:975-081.
21. Rastan A, Tepe G, Krankenberg H, et al. Sirolimus-eluting stents vs. bare-metal stents for treatment of focal lesions in infrapopliteal arteries: a double-blind, multi-centre, randomized clinical trial. Eur Heart J. 2011;32:2274–2281.
22. Bosiers M, Scheinert D, Peeters P, et al. Randomized comparison of everolimus-eluting versus bare-metal stents in patients with critical limb ischemia and infrapopliteal arterial occlusive disease. J Vasc Surg. 2012;55:390–398.
23. Scheinert D, Katsanos K, Zeller T, et al. A prospective randomized multicenter comparison of balloon angioplasty and infrapopliteal stenting with the sirolimus-eluting stent in patients with ischemic peripheral arterial disease: 1-year results from the ACHILLES trial. J Am Coll Cardiol. 2012;60:2290–2295.
24. Katsanos K, Spiliopoulos S, Diamantopoulos A, Karnabatidis D, Sabharwal T, Siablis D. Systematic review of infrapopliteal drug-eluting stents: a meta-analysis of randomized controlled trials. Cardiovasc Intervent Radiol. 2013;36:645–658.
25. Liistro F, Porto I, Angioli P, et al. Drug-eluting balloon in peripheral intervention for below the knee angioplasty evaluation (DEBATE-BTK): A randomized trial in diabetic patients with critical limb ischemia. Circulation. 2013;128:615-621.


















