Comparison of Surgical Pericardial Drainage With Percutaneous Catheter Drainage for Pericardial Effusion
Abstract: Objective. We sought to investigate the outcomes for different treatments of pericardial effusions. Background. The optimal initial management for symptomatic pericardial effusions remains controversial. Methods. We performed a 3-year retrospective, single-institution study comparing open surgical drainage to percutaneous pericardiocentesis for symptomatic pericardial effusions. Results. Between 2007 and 2009, a total of 193 patients underwent an initial drainage procedure for a pericardial effusion (n = 121 [62.7%] pericardiocentesis; n = 72 [37.3%] open surgical drainage). Compared to those treated with pericardiocentesis, treatment with open surgical drainage was associated with a higher complication rate (4.9% vs 26.4%; P<.0001; odds ratio [OR], 6.9; 95% confidence interval [CI], 2.6-18.2). Treatment with pericardiocentesis was associated with a higher rate of repeat procedures to drain a recurrent effusion compared to open surgical drainage (28.9% vs 2.8%; P<.0001; OR, 14.2; 95% CI, 3.3-61.3). Thirty-day mortality (19.8% surgical group vs 18.1% pericardiocentesis group; P=.8) and long-term survival (P=.4) did not differ between the groups. Conclusion. There is no significant difference in overall mortality between open surgical drainage and percutaneous pericardiocentesis for symptomatic pericardial effusions. There may be more procedural complications following surgical drainage of a pericardial effusion, and a greater need for repeat procedures if the effusion is drained using pericardiocentesis.
J INVASIVE CARDIOL 2012;24(11):590-593
Key words: pericardial effusion, pericardiocentesis, pericardiotomy
_______________________________________________________
Pericardial effusions are a potentially dangerous accumulation of fluid in the pericardial space that can lead to cardiac tamponade, shock, and sometimes death. The optimal therapy for symptomatic pericardial effusions remains controversial. In general, there are surgical-based approaches and percutaneous-based approaches to pericardial fluid drainage. A surgical subxiphoid approach for draining a pericardial effusion was first described in 1829,1 and there have been several additional methods proposed for surgical pericardial effusion drainage since that time.2-7 In 1986, Kopecky and colleagues reported the first percutaneous pericardiocentesis series with multiple subsequent reports characterizing the relative safety and efficacy of a percutaneous approach.8-13
We therefore aimed to compare the procedural and long-term outcomes among patients treated with percutaneous pericardial catheter drainage and patients treated with open surgical pericardial drainage for symptomatic pericardial effusions.
Methods
We reviewed the medical records of consecutive patients who underwent drainage of a symptomatic pericardial effusion at Columbia University Medical Center from January 2007 to December 2009 after appropriate ethics committee approval. Baseline demographic and clinical characteristics as well as procedural outcomes were collected from the medical record. All patients included in this study underwent either open surgical pericardial drainage (n = 72) or percutaneous pericardial catheter drainage (n = 121) as the initial procedure as determined by the clinical team caring for the patient. Chart reviews were performed by reviewers blinded to the results of the corresponding treatment arm.
A procedure was defined as urgent if it met any of the following criteria: (1) the procedure was deemed to be urgent by the primary team and documented in the patient’s chart; (2) the patient was hemodynamically unstable; (3) the patient had respiratory compromise; or (4) the effusion was related to a procedural complication (eg, perforation of a coronary during coronary intervention, perforation of myocardium during an electrophysiological procedure, postoperative bleeding) and deemed urgent by the procedural physician.
Surgical techniques. Each of the surgical techniques used for surgical drainage has been previously described in the literature; they include subxiphoid pericardiostomy, video-assisted thorascopic pericardiotomy, pericardiotomy via sternotomy, and pericardiotomy via thoracotomy. The surgical procedure was chosen at the discretion of the primary managing team and the surgeon.
Pericardiocentesis technique. All pericardiocenteses were performed in the catheterization laboratory through a subxiphoid approach under fluoroscopic guidance. Briefly, after administration of 1% lidocaine to the skin and the deeper tissues of the left xiphocostal area, a needle was advanced from the left of the subxiphoid area aiming toward the left shoulder. Once the pericardial space was entered, a guidewire was introduced into the pericardial space through the needle. The needle was removed and a catheter was inserted into the pericardial sac over the guidewire. Pericardial fluid was then removed. The catheter was frequently left in place to monitor pericardial fluid drainage. The catheter was secured to the skin with 4-0 silk sutures, covered with a sterile dressing.
Statistical analysis. Baseline characteristics were compared using Fisher’s exact test of chi-squared for proportions and the student t-test for continuous variables. Logistic regression was used to estimate the association of initial treatment strategy with 30-day procedural outcomes controlling for baseline characteristics that were statistically different between groups. Actuarial survival was calculated using the Kaplan Meier method and compared by initial treatment strategy using the log rank test. Cox proportional hazards modeling was used to adjust for baseline characteristics that were statistically different between treatment groups. Statistical differences were considered significant if the probability was .05 or less.
Results
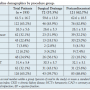 One hundred and ninety-three patients underwent a procedure for a pericardial effusion at Columbia University Medical Center from January 2007 to December 2009. The initial procedure was a percutaneous pericardiocentesis for 121 patients (62.7%) and an open surgical drainage for 72 patients (37.3%). The mean age of patients in this study was 61.5 ± 16.7 years (Table 1). There was a significant difference between the surgical and pericardiocentesis group in rates of diabetes (36.1% vs 18.2%; P<.01), recent cardiac surgery (43.1% vs 25.6%; P=.01), as well as baseline anemia (5.7% vs 5.1%; P=.02). One-hundred and twenty-six of the total procedures (65.2%) were characterized as clinically urgent, of which there was no significant difference between the surgical drainage group and pericardiocentesis group (59.7% vs 68.6%, respectively; P=.21). There was no significant difference between the groups in the remaining baseline clinical variables.
One hundred and ninety-three patients underwent a procedure for a pericardial effusion at Columbia University Medical Center from January 2007 to December 2009. The initial procedure was a percutaneous pericardiocentesis for 121 patients (62.7%) and an open surgical drainage for 72 patients (37.3%). The mean age of patients in this study was 61.5 ± 16.7 years (Table 1). There was a significant difference between the surgical and pericardiocentesis group in rates of diabetes (36.1% vs 18.2%; P<.01), recent cardiac surgery (43.1% vs 25.6%; P=.01), as well as baseline anemia (5.7% vs 5.1%; P=.02). One-hundred and twenty-six of the total procedures (65.2%) were characterized as clinically urgent, of which there was no significant difference between the surgical drainage group and pericardiocentesis group (59.7% vs 68.6%, respectively; P=.21). There was no significant difference between the groups in the remaining baseline clinical variables.
 During the procedure, the average amount of fluid removed from the pericardial space was 606.6 ± 357.9 mL, and there was no significant difference between the surgical drainage and pericardiocentesis group in the amount of fluid removed (P=.19). Among all study participants, the fluid was described as bloody in 51.9% of patients, straw-color in 12.3%, or serosanguinous in 30.1%; the fluid was not characterized in the remaining patients. The etiology of the effusion was characterized as due to malignancy in 17.1% of cases, idiopathic in 16.6%, inflammatory in 5.2%, and uremic in 3.1%, without a significant difference between the surgical drainage and pericardiocentesis group (Table 2). The etiology was characterized as postsurgical in 32.1% of cases and iatrogenic in 11.4% of cases, with significantly more patients in these groups undergoing surgical drainage vs pericardiocentesis (43.1% vs 25.6%, respectively; P=.01 and 15.7% vs 4.2%, respectively; P=.02).
During the procedure, the average amount of fluid removed from the pericardial space was 606.6 ± 357.9 mL, and there was no significant difference between the surgical drainage and pericardiocentesis group in the amount of fluid removed (P=.19). Among all study participants, the fluid was described as bloody in 51.9% of patients, straw-color in 12.3%, or serosanguinous in 30.1%; the fluid was not characterized in the remaining patients. The etiology of the effusion was characterized as due to malignancy in 17.1% of cases, idiopathic in 16.6%, inflammatory in 5.2%, and uremic in 3.1%, without a significant difference between the surgical drainage and pericardiocentesis group (Table 2). The etiology was characterized as postsurgical in 32.1% of cases and iatrogenic in 11.4% of cases, with significantly more patients in these groups undergoing surgical drainage vs pericardiocentesis (43.1% vs 25.6%, respectively; P=.01 and 15.7% vs 4.2%, respectively; P=.02).
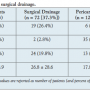 Procedural complications occurred more frequently in the surgical group than in the pericardiocentesis group (26.4% vs 4.9%; odds ratio [OR], 6.9; 95% confidence interval [CI], 2.6-18.2; P<.0001) (Table 3). Repeat drainage procedures occurred more frequently in the pericardiocentesis group (28.9% vs 2.8%; OR, 14.2; 95% CI, 3.3-61.3; P<.0001). Finally, there was no difference in 30-day mortality following pericardial effusion drained surgically or percutaneously (19.8% surgical group vs 18.1% pericardiocentesis group; P=.8) (Figure 1). After adjusting for diabetes, baseline hematocrit, size of the effusion, presence of
Procedural complications occurred more frequently in the surgical group than in the pericardiocentesis group (26.4% vs 4.9%; odds ratio [OR], 6.9; 95% confidence interval [CI], 2.6-18.2; P<.0001) (Table 3). Repeat drainage procedures occurred more frequently in the pericardiocentesis group (28.9% vs 2.8%; OR, 14.2; 95% CI, 3.3-61.3; P<.0001). Finally, there was no difference in 30-day mortality following pericardial effusion drained surgically or percutaneously (19.8% surgical group vs 18.1% pericardiocentesis group; P=.8) (Figure 1). After adjusting for diabetes, baseline hematocrit, size of the effusion, presence of  tamponade on echocardiogram, and recent cardiac surgery, the difference in mortality between pericardiocentesis and surgical drainage remained nonsignificant (hazard ratio [HR], 0.69; 95% CI, 0.31-1.54; P=.37). Length of hospitalization was shorter in the pericardiocentesis group than in the surgical drainage group (17.8 ± 28.6 days vs 26.8 ± 28.8 days, respectively; P=.04).
tamponade on echocardiogram, and recent cardiac surgery, the difference in mortality between pericardiocentesis and surgical drainage remained nonsignificant (hazard ratio [HR], 0.69; 95% CI, 0.31-1.54; P=.37). Length of hospitalization was shorter in the pericardiocentesis group than in the surgical drainage group (17.8 ± 28.6 days vs 26.8 ± 28.8 days, respectively; P=.04).
Discussion
The major findings of this study are the following: in a single-center retrospective analysis, compared to patients undergoing open surgical drainage, patients who undergo percutaneous pericardiocentesis have: (1) similar short- and long-term survival; (2) a higher rate of repeat drainage; and (3) a lower rate of postprocedural complications. Therefore, choosing between open surgical drainage and percutaneous pericardiocentesis must balance the risk of procedural complications with the benefit of a more definitive procedure.
Regardless of drainage strategy, hospitalization for pericardial effusion is associated with a high 30-day procedural mortality (approximately 20%). This mortality rate is higher than previously reported,14-17 and may reflect the high rate of postsurgical (32%) and malignant (17.1%) effusions in this study. In general, the mortality associated with pericardial effusion is likely related to the underlying condition that causes the effusion and not a direct result of the effusion itself.
In this single-center study, surgical pericardial drainage was associated with a lower recurrence rate compared to pericardiocentesis. The overall recurrence rate of approximately 30% with pericardiocentesis is similar to rate of recurrence in other series.15 Consistent with prior reports, the rate of recurrence is lower after open surgical drainage than after percutaneous pericardiocentesis. However, the reduction in recurrence rate is not without cost. When compared with percutaneous pericardiocentesis, open surgical drainage is associated with a longer hospital stay and a higher complication rate. The lower complication rate associated with percutaneous drainage seen in this study contrasts with the finding of Allen and colleagues,15 who reported a higher complication rate in subjects treated with pericardiocentesis as compared to open surgical drainage. The reduction in complications resulting from pericardiocentesis observed in this study may reflect a growing familiarity with performing pericardiocentesis since prior comparisons between surgical drainage and pericardiocentesis were performed in the mid-1990s, only 10 years after Kopecky first reported the initial case series.
The most common reason for requiring drainage of a pericardial effusion at our institution was following recent cardiac surgery. The majority of post-cardiac surgery patients with effusions had undergone some form of valvular surgery, similar to previous reports.18,19 In this study, post-surgical patients were more likely to go for surgical management of a pericardial effusion, perhaps reflecting some bias toward surgical management in these patients.
Study limitations. There are several limitations to this study. As a retrospective analysis, the study is inherently subject to confounding and bias. Also, as a single-center study, the conclusions may not reflect a broader experience with pericardiocentesis and surgical drainage for pericardial effusions. In addition, for this study, all surgical techniques were grouped together and compared to pericardiocentesis, where specific differences may exist between the techniques themselves and the operators who perform them.
Conclusion
In this study, there was no significant difference in overall mortality between open surgical drainage and percutaneous pericardiocentesis for symptomatic pericardial effusions. However, there does appear to be inherent differences between the techniques with more patients experiencing a procedural complication following surgical drainage of a pericardial effusion, and a greater need for repeat procedures if the effusion is drained using pericardiocentesis. These differences, in the appropriate clinical setting, may shape a clinician’s management of a symptomatic pericardial effusion.
References
- Larrey EL. New surgical procedure to open the pericardium in the case of fluid in the cavity. Clin Chir. 1829;36:303-337.
- Vaitkus PT, Herrmann HC, LeWinter MM. Treatment of malignant pericardial effusion. JAMA. 1994;272(1):59-64.
- Press O, Livingston R. Management of pericardial effusion and tamponade. JAMA. 1987;257(8):1088-1092.
- Miller JI, Mansour KA, Hatcher CR. Pericardiectomy: current indications, concepts and results in a university center. Ann Thorac Surg. 1982;34(1):40-45.
- Piehler JM, Pluth JR, Schaff HV, et al. Surgical management of effusive pericardial disease: influence of extent of pericardial resection on clinical course. J Thorac Cardiovasc Surg. 1985;90(4):506-516.
- Hazelrigg SR, Mack MJ, Landreneau RJ, et al. Thoracoscopic pericardiectomy for effusive pericardial disease. Ann Thorac Surg. 1993;56(3):792-795.
- Shapira OM, Aldea GS, Fonger JD, Shemin RJ. Video-assisted thoracic surgical techniques in the diagnosis and management of pericardial effusion in patients with advanced lung cancer. Chest. 1993;104(4):1262-1263.
- Kopecky SL, Callahan JA, Tajik J, Seward JB. Percutaneous pericardial catheter drainage: report of 42 consecutive cases. Ann J Cardiol. 1986;58(7):633-635.
- Celermajer DS, Boyer MJ, Bailey BP, Tattersall MHN. Pericardiocentesis for symptomatic malignant pericardial effusion: a study of 36 patients. Med J Aust. 1991;154(1):19-22.
- Ziskind AA, Pearce C, Lemmon CC, et al. Percutaneous balloon pericardiotomy for the treatment of cardiac tamponade and large pericardial effusions: description of technique and report of the first 50 cases. J Am Coll Cardiol. 1993;21(1):1-5.
- Lemmon CC, Ziskind AA. Percutaneous balloon pericardiotomy: nonsurgical alternative for treating malignancy-related pericardial effusions. Card B Rev. 1993;10(1):53-59.
- Di Segni E, Lavee J, Kaplinsky E, Vered Z. Percutaneous balloon pericardiostomy for the treatment of cardiac tamponade. Eur Heart J. 1995;16(2):184-187.
- Shepherd FA, Morgan C, Evans WK, Ginsberg JF, Watt D, Murphy K. Medical management of malignant pericardial effusion by tetracycline sclerosis. Am J Cardiol. 1987;60(14):1161-1166.
- Davis S, Rambotti P, Grignani F. Intrapericardial tetracycline sclerosis in the management of malignant pericardial effusion: an analysis of thirty-three cases. J Clin Oncol. 1984;2(6):631-636.
- Allen KB, Faber LP, Warren WH, Shaar CJ. Pericardial effusion: subxiphoid pericardiostomy versus percutaneous catheter drainage. Ann Thorac Surg. 1999;67(2):437-440.
- Becit N, Unlü Y, Ceviz M, Koçogullari CU, Koçak H, Gürlertop Y. Subxiphoid pericardiostomy in the management of pericardial effusions: case series analysis of 368 patients. Heart. 2005;91(6):785-790.
- Susini G, Pepi M, Sisillo E, et al. Percutaneous pericardiocentesis versus subxiphoid pericardiotomy in cardiac tamponade due to postoperative pericardial effusion. Cardiothorac Vasc Anesth. 1993;7(2):178-183.
- Mangi AA, Palacios IF, Torchiana DF. Catheter pericardiocentesis for delayed tamponade after cardiac valve operation. Ann Thorac Surg. 2002;73(5):1479-1483.
- Kuvin JT, Harati NA, Pandian NG, Bojar RM, Khabbaz KR. Postoperative cardiac tamponade in the modern surgical era. Ann Thorac Surg. 2002;74(4):1148-1153.
________________________________________________________
From the 1Cardiology Division, Massachusetts General Hospital, Boston, Massachusetts, 2Department of Medicine, Columbia University Medical Center, New York, New York, 3Cardiovascular Research Foundation, New York, New York, 4Department of Surgery, Columbia University Medical Center, New York, New York, 5Cardiology Division and Center for Interventional Vascular Therapy, Columbia University Medical Center, New York, New York, and the 6Division of Cardiology, Mount Sinai School of Medicine, New York, New York.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted April 5, 2012, provisional acceptance given May 2, 2012, final version accepted May 7, 2012.
Address for correspondence: Adam J. Saltzman, MD, Massachusetts General Hospital, Division of Cardiology, 55 Fruit Street, Blake 950, Boston, MA 02114. Email: ajsaltzman@partners.org


















