Comparison of Radial Access, Guided Femoral Access, and Non-Guided Femoral Access Among Women Undergoing Percutaneous Coronary Intervention
Abstract: Objectives. This study was conducted to determine the association between radial access, guided femoral access, and non-guided femoral access on postprocedural bleeding and vascular complications after percutaneous coronary intervention (PCI). Background. Bleeding events and major vascular complications after PCI are associated with increased morbidity, mortality, and cost. While the radial approach has been shown to be superior to the femoral approach in reducing bleeding and vascular complications, whether the use of micropuncture, fluoroscopy, or ultrasound mitigates these differences is unknown. Methods. We conducted a post hoc analysis of women in the SAFE-PCI for Women trial who underwent PCI and had the access method identified (n = 643). The primary endpoint of postprocedure bleeding or vascular complications occurring within 72 hours or at discharge was adjudicated by an independent clinical events committee and was compared based on three categories of access technique: radial, guided femoral (fluoroscopy, micropuncture, ultrasound), or non-guided femoral (none of the aforementioned). Differences between the groups were determined using multivariate logistic regression using radial access as the reference. Results. Of the PCI population, 330 underwent radial access, 228 underwent guided femoral access, and 85 underwent non-guided femoral access. There was a statistically significant lower incidence of the primary endpoint with radial access vs non-guided femoral access; however, there was no significant difference between radial approach and femoral access guided by fluoroscopy, micropuncture, or ultrasound. Conclusions. This post hoc analysis demonstrates that while radial access is safer than non-guided femoral access, guided femoral access appears to be associated with similar bleeding events or vascular complications as radial access.
J INVASIVE CARDIOL 2018;30(1):18-22. Epub 2017 October 15.
Key words: radial access, vascular complications, bleeding
Radial access has been shown to improve outcomes in populations at higher risk of bleeding, such as the elderly, women, and patients presenting with acute coronary syndromes (ACS). However, despite these data, femoral access continues to be the most common access site for percutaneous coronary intervention (PCI) in many countries, including the United States. One reason underlying the reluctance to adopt radial access may be the belief that “guided” femoral access1 – the use of ultrasound, femoral-head fluoroscopy, and/or micropuncture – achieves the same reduction in bleeding and vascular complications as radial access. Available studies have produced conflicting results when comparing these methods used to obtain femoral access;2-4 no study has compared the specific methods of acquiring femoral access with radial access when evaluating postprocedure bleeding and vascular complications. Accordingly, we used data from the SAFE-PCI for Women trial to determine the association between radial access, guided femoral access, and non-guided femoral access on postprocedural bleeding and vascular complications after PCI.
Methods
The SAFE-PCI for Women trial. The SAFE-PCI for Women trial study design and primary results have been previously described.5,6 Briefly, the trial was a multicenter, prospective, open-label, randomized, controlled clinical trial that was conducted to compare the efficacy and feasibility of radial access with femoral access in women undergoing coronary angiography or PCI. The trial randomized 1787 women undergoing diagnostic cardiac catheterization or PCI to either radial or femoral access. Women were eligible for the trial if they were older than 18 years of age, able to provide informed consent, and undergoing evaluation or treatment for ischemic heart disease. Exclusion criteria included conditions precluding arterial access in either the femoral or radial artery (peripheral arterial disease severe enough to preclude arterial access, absence of collateral flow in both hands assessed by use of Allen or Barbeau7 test, active hemodialysis fistula, or graft in an arm to be used in case of assignment to radial access), international normalized ratio ≥1.5 in the presence of ongoing treatment with vitamin K antagonists, receipt of an oral factor IIa or Xa inhibitor in the 24 hours before the PCI procedure, known valvular heart disease requiring valve surgery, planned right heart catheterization, primary PCI for ST-segment elevation myocardial infarction (STEMI), known presence of bilateral internal mammary coronary bypass grafts, participation in an investigational drug or device study within 30 days before enrollment, and planned staged PCI within 30 days after index PCI.
The study was terminated early due to a lower than expected event rate and no significant difference was found in the primary efficacy endpoint of Blood Academic Research Consortium (BARC) types 2, 3, or 5 bleeding or major vascular complications at 72 hours post procedure between radial and femoral access in women undergoing PCI; among women undergoing angiography or PCI, radial access significantly reduced the primary outcome by 60%.6
The Duke University Institutional Review Board approved the current analysis and provided a waiver of informed consent as this is a post hoc analysis of prospectively collected clinical trial data.
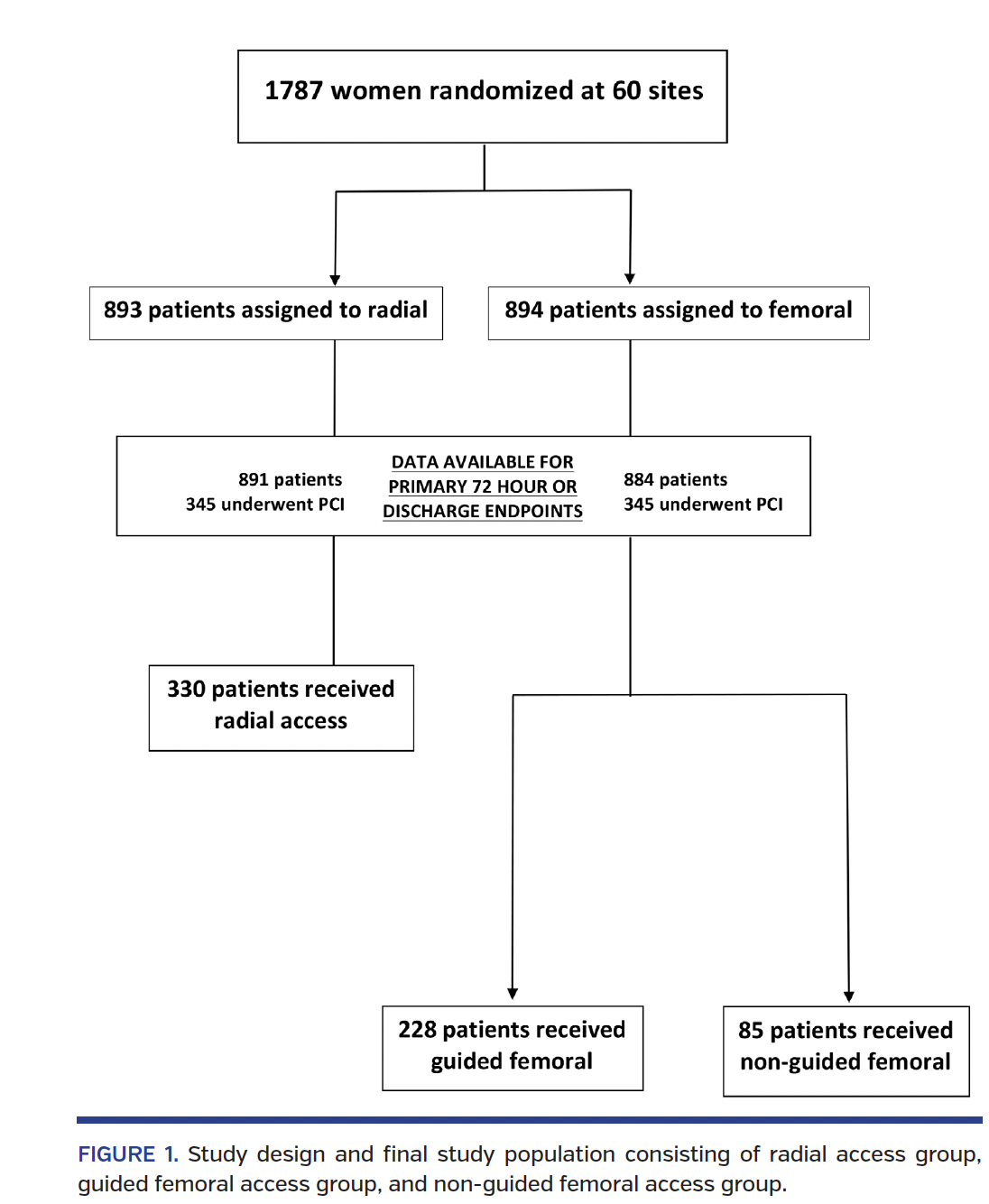
Study sample. For the present analysis, we excluded any patient who did not undergo PCI (n = 1096) or did not have information on access strategy (n = 48) (Figure 1). Consequently, the study population consisted of 643 subjects who underwent PCI and had access method identified.
Treatment strategies. Specific antiplatelet and anticoagulant regimens for PCI were not mandated, but general and access-specific recommendations were included. This included aspirin and a P2Y12 inhibitor at the discretion of the investigator in the trial. For all PCI procedures, bivalirudin was recommended; glycoprotein IIb/IIIa inhibitors were allowed on a discretionary basis, but up-front intention to use a glycoprotein inhibitor was specified prior to randomization. Postdischarge antiplatelet duration was also left to the operator, but must have been for at least 30 days in stented patients. Similarly, aside from randomized assignment to radial or femoral access, the specific technique of obtaining access was left to the discretion of the operator. The radial artery was accessed by either counterpuncture (“through and through”) or anterior-wall puncture techniques. For femoral artery access, the use of micropuncture or fluoroscopic or ultrasound guidance was recommended but not mandated. In addition, duration of manual pressure for hemostasis and use of vascular closure devices were in accordance with local site practice.
Endpoints. The primary efficacy endpoint was bleeding or vascular complications requiring intervention occurring within 72 hours of the procedure or by hospital discharge, whichever came first. Bleeding was defined according to the BARC definitions,8 and the endpoint of interest for the study included a composite of BARC type 2, 3, or 5 bleeding events. Vascular complications were defined as any of the following that required surgical intervention, including thrombin injection: arteriovenous fistula, arterial pseudoaneurysm, or arterial occlusion. All endpoints were adjudicated by an independent clinical events committee using standardized definitions.5
Statistical analysis. This post hoc analysis evaluates the subgroup of women who underwent PCI in the SAFE-PCI for Women trial who also had the method of access identified. Patients were grouped according to initial access-site strategy, ie, radial access, guided femoral access, or non-guided femoral access. Continuous variables are expressed as median (25th, 75th percentiles) and categorical variables are expressed as percentages. To determine the association between access strategy and outcome, we performed multivariate logistic regression with primary efficacy endpoint as the outcome. The models incorporated planned use of glycoprotein IIa/IIIb inhibitors during PCI, and elective versus ACS indication for PCI as covariates for adjustment and access method. A P-value of <.05 was required for statistical significance. Statistical analyses were performed by SAS version 9.
Results
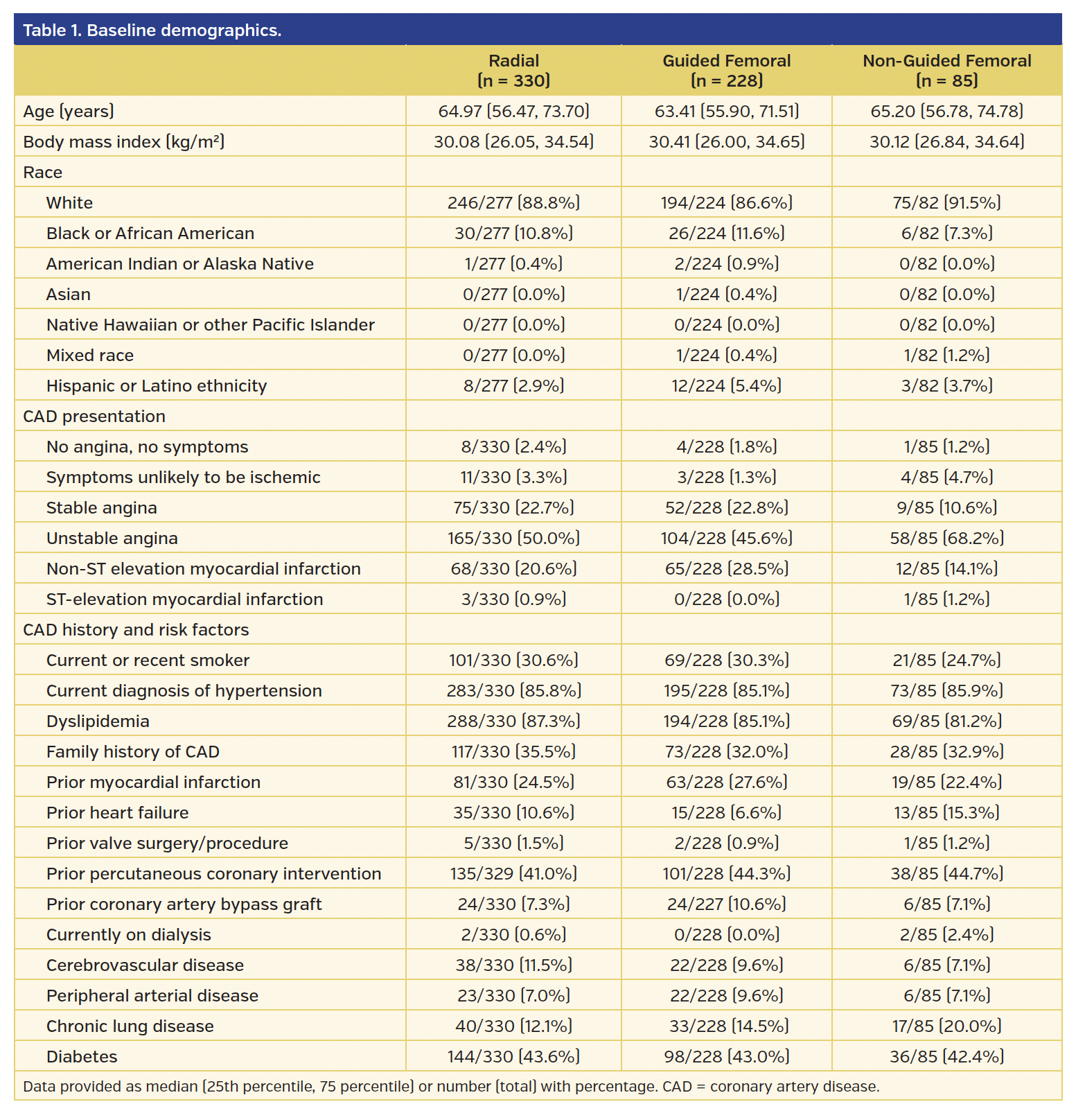
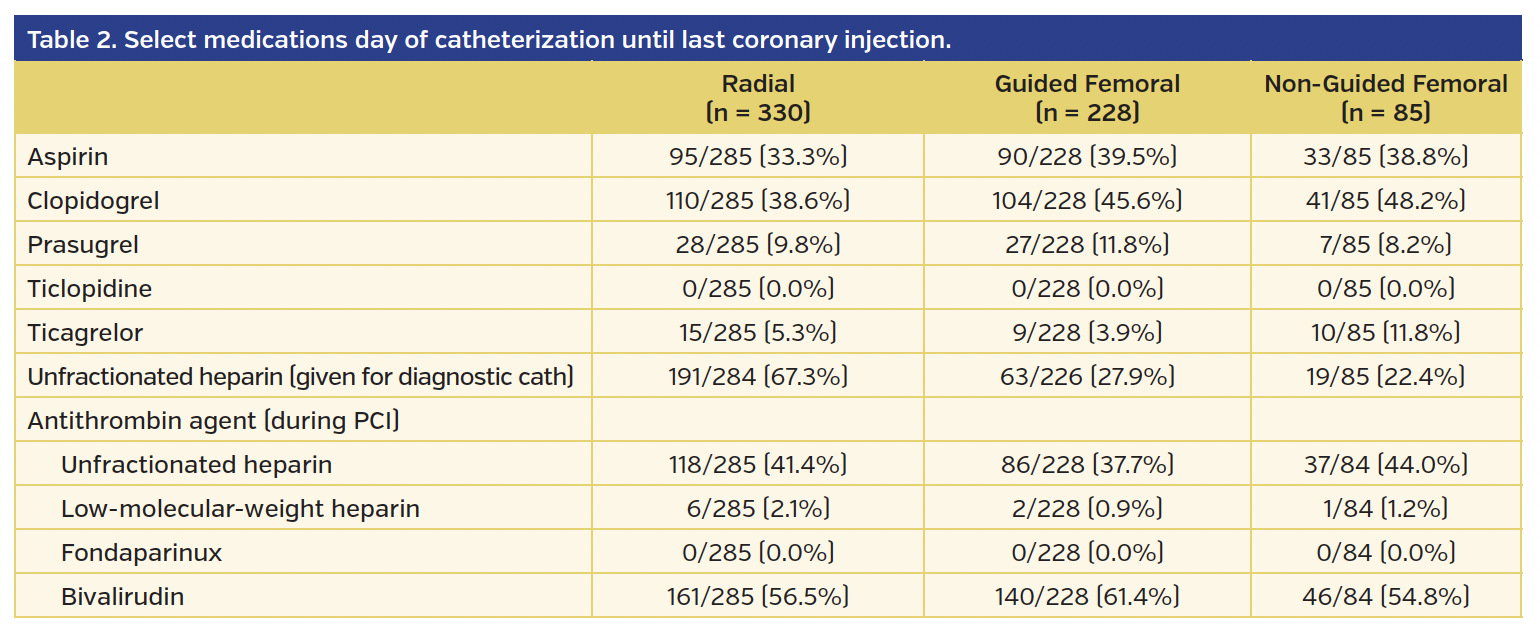
From the original study population of 1787 women in the SAFE-PCI for Women trial, there were 691 subjects who underwent PCI. Those who did not have access method identified (n = 48) were excluded from the analysis. Of the 643 subjects, 330 underwent radial access, 228 underwent guided femoral access, and 85 underwent non-guided femoral access. Of the guided femoral access group, fluoroscopy was used in 152 subjects, ultrasound in 1 subject, and micropuncture in 45 subjects (there was also overlap where providers used multiple modalities). Baseline demographics for each group are presented in Table 1. The majority of procedures were performed for non-STEMI or unstable angina. Unfractionated heparin was used during the procedure in 41.4% of the radial group, 37.7% of the guided femoral group, and 44.0% of the non-guided femoral group. Bivalirudin was used during 56.5% of procedures in the radial group, 61.4% of the guided femoral group, and 54.8% in the non-guided femoral group (Table 2). The use of glycoprotein IIb/IIIa inhibitors during PCI was minimal and similar across all groups.
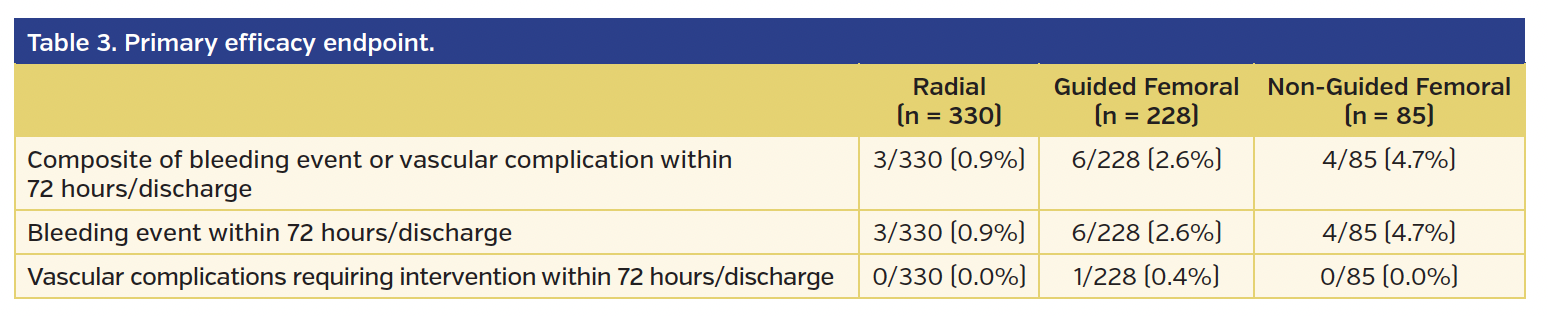
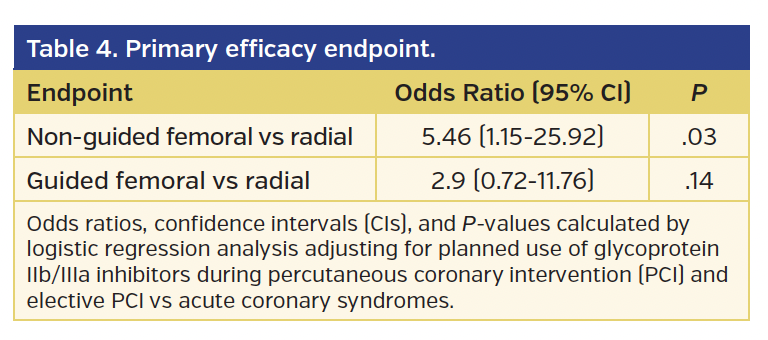
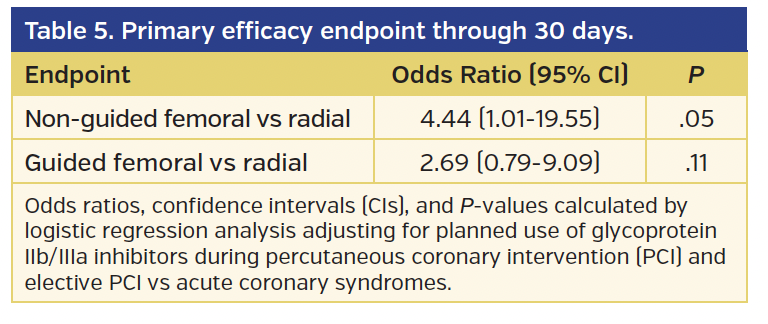
The rates of BARC type 2, 3, or 5 bleeding or vascular complications requiring intervention (the primary endpoint of the study) were significantly higher in the non-guided femoral group vs the radial group (4.7% vs 0.9%, respectively; odds ratio [OR], 5.46; 95% confidence interval [CI], 1.15-25.92; P=.03) (Tables 3 and 4). However, while there was a numerical difference, there was no statistically significant difference in the primary endpoint comparing the guided femoral group vs the radial group (2.6% vs 0.9%, respectively; OR, 2.90; 95% CI, 0.72-11.76; P=.14). At 30-day follow-up, the lower incidence of the primary endpoint in the radial access group vs the non-guided femoral access group was of borderline statistical significance (P=.05) (Table 5).
Discussion
The main findings of the present analysis of women undergoing PCI by either the radial, guided femoral, or non-guided femoral route demonstrated that there were fewer bleeding and vascular complications in the radial group when compared with the non-guided femoral group. This difference was of borderline significance at 30-day follow-up. Importantly, there was no statistically significant difference in complications between the radial group and the guided femoral group. These data suggest that while the radial approach may be safer than the femoral approach in women, the use of “guided” femoral access may attenuate the difference. These data support the need for a randomized trial comparing guided femoral access with radial access.
Previous studies evaluating access methods have shown inconsistent findings. For example, two studies comparing standard 18 gauge needles to the 21 gauge (micropuncture) needle found no significant  difference for the primary endpoint of femoral vascular complications.2,3 In the FAUST trial, ultrasound-guided access compared to fluoroscopy-guided access demonstrated that fluoroscopy-guided access of the femoral artery resulted in increased hematoma formation compared to ultrasound-guided in obtaining femoral access.4 Because there is no formal recommendation for which method to use, operators subsequently obtain femoral access based on their preference or that of their institution. As the safety of cardiac catheterization vis-à-vis ischemic events has improved, there is increased emphasis on reducing the risks of undergoing these procedures. The results of this current analysis suggest that operators should strongly consider using fluoroscopic or ultrasound guidance for femoral access. This may be particularly important for procedures requiring large-bore arterial access like transcatheter aortic valve replacement or hemodynamic support.9
difference for the primary endpoint of femoral vascular complications.2,3 In the FAUST trial, ultrasound-guided access compared to fluoroscopy-guided access demonstrated that fluoroscopy-guided access of the femoral artery resulted in increased hematoma formation compared to ultrasound-guided in obtaining femoral access.4 Because there is no formal recommendation for which method to use, operators subsequently obtain femoral access based on their preference or that of their institution. As the safety of cardiac catheterization vis-à-vis ischemic events has improved, there is increased emphasis on reducing the risks of undergoing these procedures. The results of this current analysis suggest that operators should strongly consider using fluoroscopic or ultrasound guidance for femoral access. This may be particularly important for procedures requiring large-bore arterial access like transcatheter aortic valve replacement or hemodynamic support.9
Study limitations. There are several limitations to this analysis. First, since this is a post hoc analysis, any significant findings are hypothesis generating, but were not the original intent of the study. The results can be used to develop future studies for definitive answers comparing specific methods of femoral access to radial access. Additionally, loss of randomization occurred once women in the femoral arm were further stratified into guided and non-guided approaches. As a result, potential confounders that were presumably controlled for in the randomized setting were not controlled for in this post hoc analysis. Finally, in the original SAFE-PCI for Women trial, the use of fluoroscopy or ultrasound guidance was recommended but not mandated in the femoral arm. Thus, the preference for a specific method of access was probably dependent on the operator or institution at which the PCI was being performed. Concerns have been raised that as institutions transition to radial access as the primary access site for cardiac catheterization, providers will become less proficient in femoral access. Reassuringly, recent data have found that once differences in case mix across centers are adjusted for, femoral outcomes are similar between high and low radial proportion centers.10
Conclusion
In this post hoc analysis from the SAFE-PCI for Women trial, there were fewer bleeding complications with radial access when compared with non-guided femoral access (without the use of micropuncture, ultrasound, or fluoroscopy). In addition, guided access appears safer than non-guided femoral access. Future studies will need to examine how radial access compares with specific methods of guided femoral access in reducing bleeding and vascular complications.
References
1. Bangalore S, Bhatt DL. Femoral arterial access and closure. Circulation. 2011;124:e147-e156.
2. Ambrose JA, Lardizabal J, Mouanoutoua M, et al. Femoral micropuncture or routine introducer study (FEMORIS). Cardiology. 2014;129:39-43.
3. Ben-Dor I, Maluenda G, Mahmoudi M, et al. A novel, minimally invasive access technique versus standard 18-gauge needle set for femoral access. Catheter Cardiovasc Interv. 2012;79:1180-1185.
4. Seto AH, Abu-Fadel MS, Sparling JM, et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With Ultrasound Trial). JACC Cardiovasc Interv. 2010;3:751-758.
5. Hess CN, Rao SV, Kong DF, et al. Embedding a randomized clinical trial into an ongoing registry infrastructure: unique opportunities for efficiency in design of the Study of Access site For Enhancement of Percutaneous Coronary Intervention for Women (SAFE-PCI for Women). Am Heart J. 2013;166:421-428.
6. Rao SV, Hess CN, Barham B, et al. A registry-based randomized trial comparing radial and femoral approaches in women undergoing percutaneous coronary intervention: the SAFE-PCI for women (Study of Access Site for Enhancement of PCI for Women) trial. JACC Cardiovasc Interv. 2014;7:857-867.
7. Barbeau GR, Arsenault F, Dugas L, Simard S, Lariviere MM. Evaluation of the ulnopalmar arterial arches with pulse oximetry and plethysmography: comparison with the Allen’s test in 1010 patients. Am Heart J. 2004;147:489–493.
8. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736-2747.
9. Généreux P, Cohen DJ, Mack M, et al. Incidence, predictors, and prognostic impact of late bleeding complications after transcatheter aortic valve replacement. J Am Coll Cardiol. 2014;64:2605-2615.
10. Hulme W, Sperrin M, Kontopantelis E, et al; for the British Cardiovascular Intervention Society and the National Institute of Cardiovascular Outcomes Research. Increased radial access is not associated with worse femoral outcomes for percutaneous coronary intervention in the United Kingdom. Circ Cardiovasc Interv. 2017;10:e004279.
From the 1Duke University Medical Center, Durham, North Carolina; 2Duke Clinical Research Institute, Durham, North Carolina; 3University of Colorado Denver, Aurora, Colorado; 4Ohio State University Medical Center, Colombus, Ohio; 5McMaster University, Hamilton, Ontario, Canada; 6Boston Medical Center, Boston, Massachusetts; 7Harvard Medical School, Boston, Massachusetts; 8Mount Sinai Hospital, Chicago, Illinois; 9Penn State Heart and Vascular Institute, Hershey, Pennsylvania; and 10Durham VA Medical Center, Durham, North Carolina.
Funding: Duke Clinical Research Institute, Durham, North Carolina. Terumo Interventional Systems provided partial support for SAFE-PCI.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Jacobs is the site PI for the Absorb stent studies for Abbott Vascular. Dr Krucoff reports research grants and moderate consulting for Terumo, Medtronic, Abbott Vascular, Boston Scientific, Acist, Eli Lilly, and Sanofi. Dr Jolly reports grant support from Medtronic, Boston Scientific; speaker fees from Astra Zeneca. Dr Gibson reports grant support from Angel Medical, Bayer, CSL Behring, Ikaria, Janssen Pharmaceuticals, Johnson & Johnson, and Portola Pharmaceuticals; consultant for Bayer, The Medicines Company, Boston Clinical Research Institute, Cardiovascular Research Foundation, Eli Lilly, Gilead Sciences, Novo Nordisk, Pfizer, WebMD, and UpToDate in Cardiovascular Medicine. Dr Mehran reports grants paid to her institution from AstraZeneca, Bayer, Beth Israel Deaconess, BMS, CSL Behring, Eli Lilly/DSI, Medtronic, Novartis Pharmaceuticals, and Orbus Neich; consultant fees (paid to institution) from Abbott Laboratories, CardioKinetix, Spectranetics, and Watermark Research Partners; consultant fees (paid to spouse) from Abiomed and The Medicines Company; consultant fees (personal) from Boston Scientific, CSI, Medscape, Shanghai BraccoSine Pharmaceuticals, and AstraZeneca; <1% equity in Claret Medical and Elixir Medical; and executive committee for Janssen Pharmaceuticals and Osprey Medical. Dr Gilchrist reports consulting for Terumo Interventional Systems. Dr Rao reports consultant fees from Medtronic. The remaining authors report no conflicts of interest regarding the content herein.
Manuscript submitted May 14, 2017, provisional acceptance given May 18, 2017, final version accepted July 3, 2017.
Address for correspondence: Linda M. Koshy, MD, Medical Education Program, Department of Medicine, Duke University Medical Center, DUMC Box 3182, Durham, NC 27710. Email: linda.koshy@duke.edu
















