Clinical and Echocardiographic Features Associated With Improved Survival in Patients With Severe Aortic Stenosis Undergoing Balloon Aortic Valvuloplasty (BAV)
Abstract: Background. Balloon aortic valvuloplasty (BAV) is used in high-risk patients with severe aortic stenosis (AS) when the benefit of transcatheter aortic valve replacement (TAVR) or surgical aortic valve replacement (SAVR) is unclear. Our objective was to identify clinical or echocardiographic features that identify patients likely to benefit from BAV. Methods. We studied 141 consecutive patients who underwent BAV from July, 2011 to October, 2017. Clinical characteristics, routine echocardiographic parameters, and speckle tracking imaging of global longitudinal strain (GLS) were assessed before and after BAV. The primary outcome was all-cause mortality as ascertained by the National Death Index. Results. There were 141 patients, median age, 80 years (interquartile range [IQR], 74-87 years) with severe AS (median aortic valve area, 0.66 cm2; IQR, 0.53-0.79 cm2) and median mean gradient of 36 mm Hg (IQR, 27-48 mm Hg) who underwent BAV. The 1-year mortality rate was 52%. Characteristics associated with survival were New York Heart Association class I symptoms, lower brain natriuretic peptide level, higher left ventricular ejection fraction (LVEF) >53%, and higher GLS (>13.2%; absolute values were used for GLS). Landmark analysis at 60 days showed the 47 patients who underwent TAVR/SAVR after BAV had significantly better 1-year survival than those who did not (P<.001). Conclusion. A high 1-year mortality rate was observed in severe AS patients selected for BAV. LVEF and left ventricular (LV)-GLS offer similar prognostic value for 1-year mortality; however, LV-GLS may have potentially increased clinical utility, as it provides a clear threshold for predicting poor outcomes compared with LVEF. As patients who undergo TAVR/SAVR have markedly improved mortality, careful consideration should be given to advance definitive valve therapy in carefully selected BAV patients.
Key words: aortic stenosis, balloon aortic valvuloplasty, global longitudinal strain
Balloon aortic valvuloplasty (BAV) was introduced in 1985 as an alternative to surgical aortic valve replacement (SAVR) in high-risk adult patients with severe aortic stenosis (AS).1,2 While BAV improved aortic valve hemodynamics, beneficial effects were heterogeneous, not permanent, and BAV did not improve long-term mortality.2-5 In the present era, the development of transcatheter aortic valve replacement (TAVR) has led to a more definitive improvement in symptoms, hemodynamics, and mortality in appropriately selected patients at low to prohibitive risk for surgery.6-8 However, in the subset of patients with symptomatic, severe AS, where the benefit of definitive aortic valve replacement is unclear, current clinical guidelines suggest that BAV may be used as a bridging procedure.9
There are limited data identifying clinical or imaging characteristics of patients who are likely to benefit from a BAV bridging strategy. Accordingly, our objective was to determine clinical and echocardiographic features, including global longitudinal strain (GLS), that are associated with improved mortality in patients undergoing BAV.
Methods
Study population. We studied 141 patients with severe symptomatic AS who underwent BAV from July 2011 to October 2017 at our institution. Indications for BAV included need for urgent procedure, such as cardiogenic shock, “bridge to decision” in patients with unclear symptoms, and palliative in those deemed not to be candidates for definitive valve therapy. Patients in cardiogenic shock were deemed inoperable and were treated with a BAV to mitigate hemodynamic derangements; this was done with the potential to have a future definitive valve procedure. Patients treated as a bridge to decision often had coexisting comorbidities, such as severe chronic obstructive pulmonary disease, in which it was unclear if improving their aortic valve hemodynamics would improve their symptoms. Finally, patients treated as a palliative procedure often had comorbidities — such as metastatic cancer — that precluded a definitive valve therapy and BAV was offered for symptom improvement. The study was approved by our institutional review board with a waiver of consent.
BAV procedure. All procedures were performed using local anesthesia and a transfemoral retrograde approach. Heparin (50 IU/kg) was administered after sheath insertion in the femoral artery. Mean aortic gradient was measured before and after valvuloplasty by simultaneous recording of aortic and left ventricular (LV) pressures. Using a dual-lumen catheter, cardiac output was estimated by the Fick method at baseline, and aortic valve area (AVA) was calculated using Gorlin’s formula.10 Selection of balloon size was based on the combination of transthoracic echocardiography, baseline aortography, and patient body size (Figure 1). The default balloon size was 23 mm. A 20 mm balloon was used in densely calcified valves or when the aortic annulus was <19 mm by transthoracic echocardiography. The 25 mm-diameter balloon size was used if aortic annulus diameter was >24 mm or to improve results post 23 mm BAV. To stabilize the balloon position across the valve, rapid ventricular pacing (180-220 beats/minute) was systematically used during each inflation.
Data acquisition and echocardiography methods. Clinical, laboratory, and procedural data were collected from the electronic medical records. Echocardiograms that were completed before and after BAV were collected for offline analysis. Standard echocardiographic parameters and speckle-tracking strain analyses were completed using commercially available TomTec software (2D Cardiac Performance Analysis, version 4.6; TomTec). Echocardiography investigators analyzing the images were blinded to clinical characteristics, treatment assignment, and outcomes. LV volumes were measured by Simpson’s disk method in the apical 4-chamber and 2-chamber views and LV ejection fraction (LVEF) was calculated according to the American Society of Echocardiography protocols. LV-GLS was calculated as the average strain of 18 segments from 3 standard apical views.11 GLS is described in absolute values throughout, as previously reported,12 to avoid confusion resulting from negative values.
Mortality data. The National Death Index (NDI) has been shown to be a valid marker of patient vital status13,14 and has been used in numerous cardiovascular studies.15,16 Participant names, dates of birth, and social security numbers were submitted to the NDI. For deceased participants, the NDI supplied the name, date of birth, age, sex, and date of death. Each participant’s Social Security number was used as the primary matching criterion, whereas names, dates of birth, dates of death, and state of death were used in cases of inconsistency in the reported Social Security number.
Statistical analysis. All analyses were performed using R statistical software, version 3.6. Categorical variables were compared univariately using a Chi-squared test or Fisher’s exact test for group comparisons, as appropriate. McNemar tests were used for baseline vs follow-up analysis. Most continuous variables were not normally distributed and were reported as median with interquartile range (IQR) and compared using Wilcoxon test (paired test for baseline vs follow-up). The effect of LVEF and GLS mortality was assessed using median value splits in survival analysis. Time to death (mortality) was visualized using Kaplan-Meier curves and compared using log-rank test and Cox proportional hazards models, which were adjusted for age, gender, and LVEF. Other potential covariates were assessed as well, but not included in the models due to high number of missing values and high correlation with LVEF. In the model looking at the effect of TAVR/SAVR on mortality, TAVR/SAVR was treated as a time-dependent covariate since a definitive valve procedure was performed after baseline. P-values reported as <.05 were significant.
Results
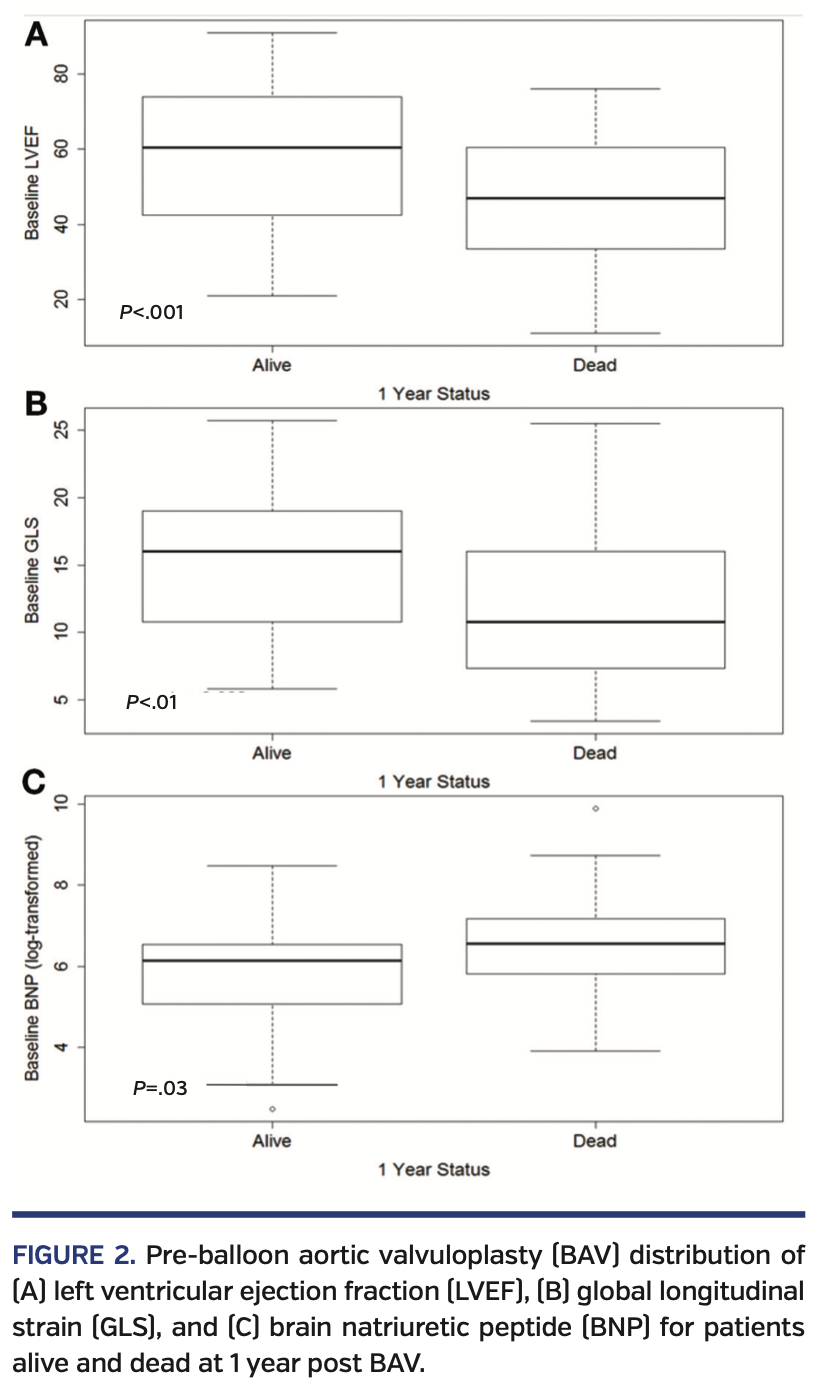
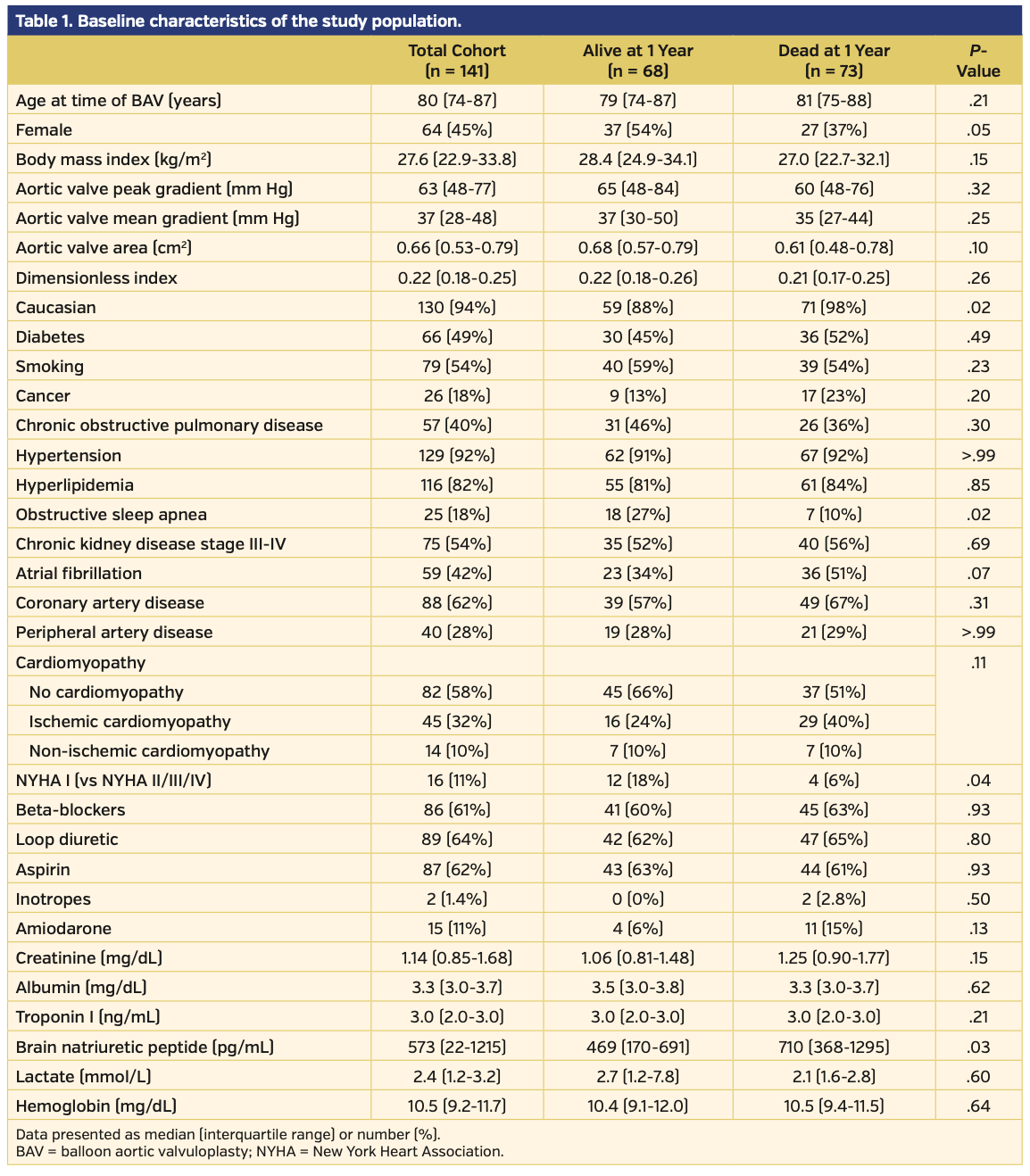
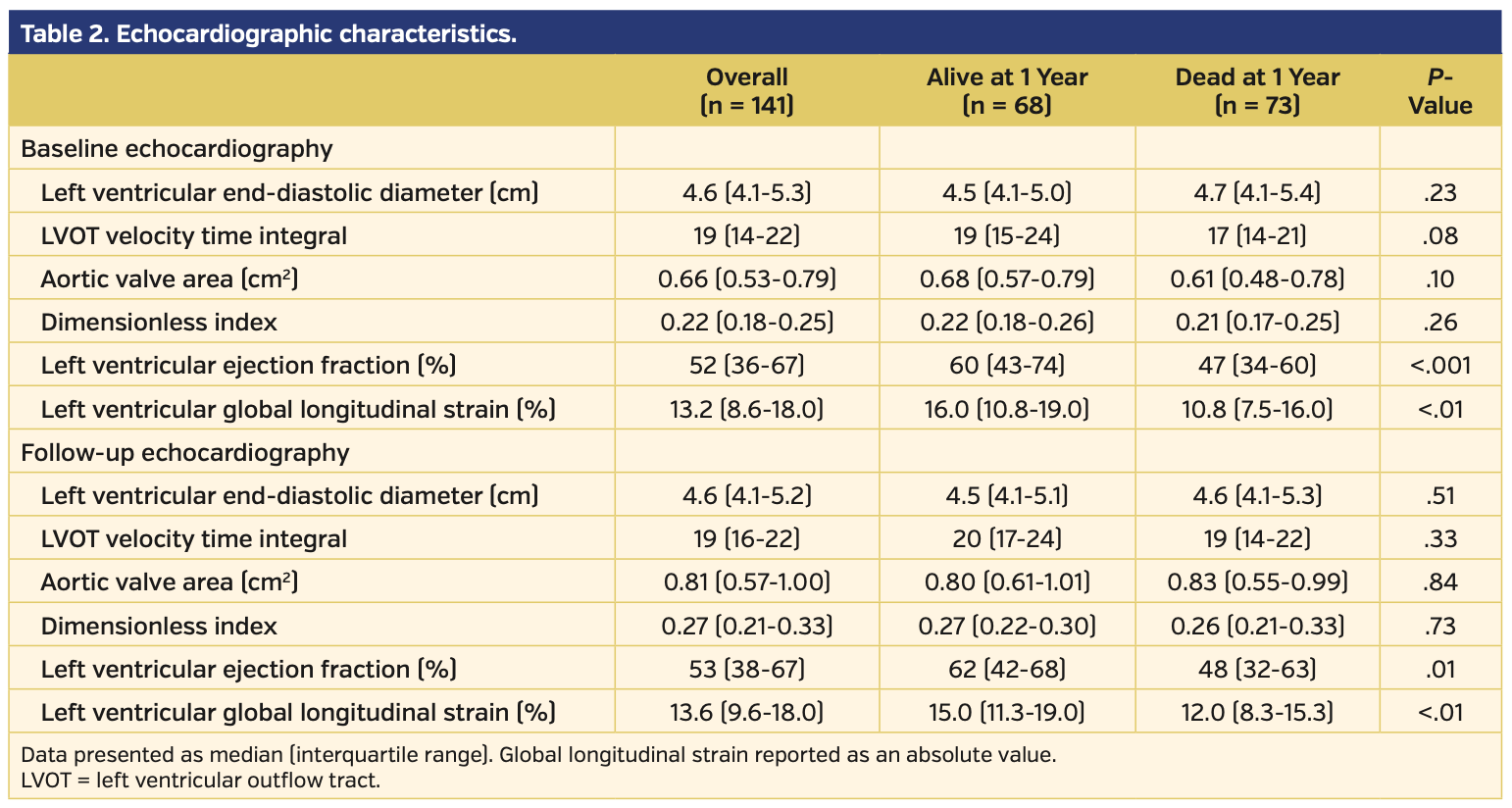
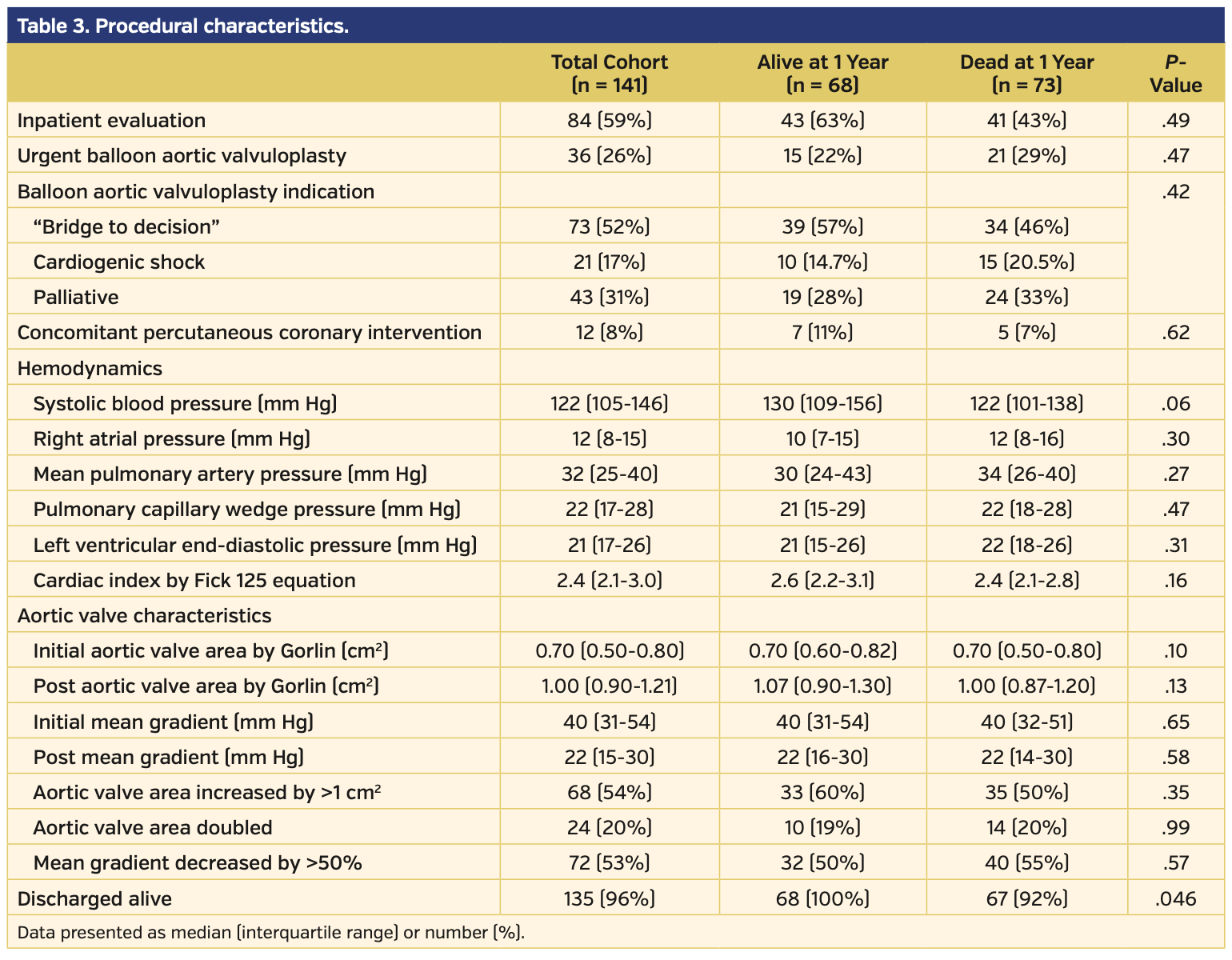
Study population. The baseline demographics for the 141 patients included in this study are presented in Table 1. Pre-BAV distribution of LVEF, GLS, and brain natriuretic peptide for patients alive and dead at 1 year post PAV are presented in Figure 2. Baseline echocardiographic data demonstrated that this cohort had severe AS (median aortic valve mean gradient [AV-MG], 37 mm Hg; median aortic valve peak gradient [AV-PG], 63 mm Hg; median AVA, 0.66 cm2; median dimensionless index [DI], 0.22) with reduced LV function (median LVEF, 53%; median LV-GLS, -13.2%) (Table 2). Procedural characteristics are presented in Table 3 and confirm severe AS (initial AV-MG, 40 mm Hg; initial AVA, 0.70 cm2), elevated filling pressures, and low cardiac index in the cohort. Overall, 52% of patients were treated as a bridge to decision, 17% presented in cardiogenic shock, and 31% of patients were considered palliative. The procedure was deemed successful in all patients; however, there were periprocedural complications in 6 patients (4.3%), including vascular complications (n = 3), complete heart block (n = 2), and stroke (n = 1). Of the 141 patients, a total of 6 (4.3%) did not survive to hospital discharge.
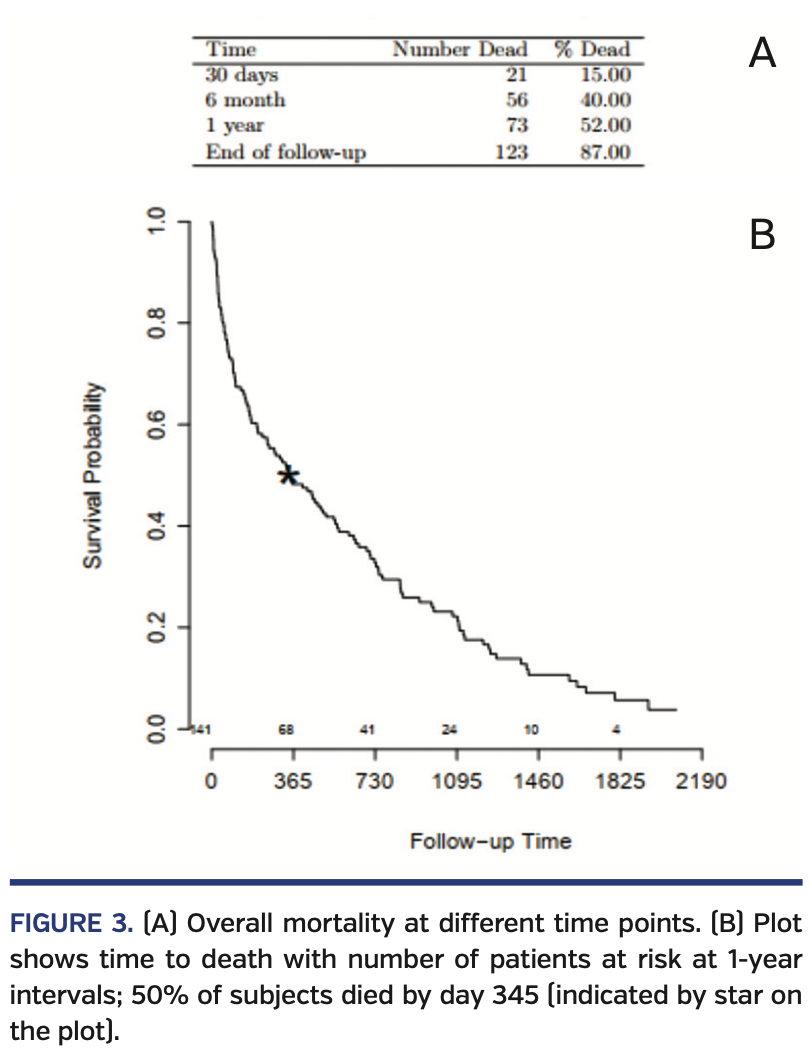
Mortality results. Following BAV, the overall 30-day mortality rate was 15% and the 1-year mortality rate was 52% (Figure 3). Compared with those who died, patients who were alive were more likely to have obstructive sleep apnea (26% vs 10%; P=.02), New York Heart Association (NYHA) class I symptoms (18% vs 5%; P=.04), a lower brain natriuretic peptide level (469 pg/mL vs 710 pg/mL; P=.03), a higher LVEF (60% vs 47%; P<.001), and a higher LV-GLS (16% vs 10.7%; P<.01; higher absolute GLS value indicates better myocardial performance). We investigated LV-GLS, LVEF, age, gender, NYHA class, and BNP as possible covariates in a multivariate model. However, due to the high number of missing values and the high correlation with LVEF values, we decided not to adjust for BNP and NYHA. The final model investigated the effect of LV-GLS, LVEF, age, and gender; only LVEF and gender were significant, with age having borderline effect (P=.07).
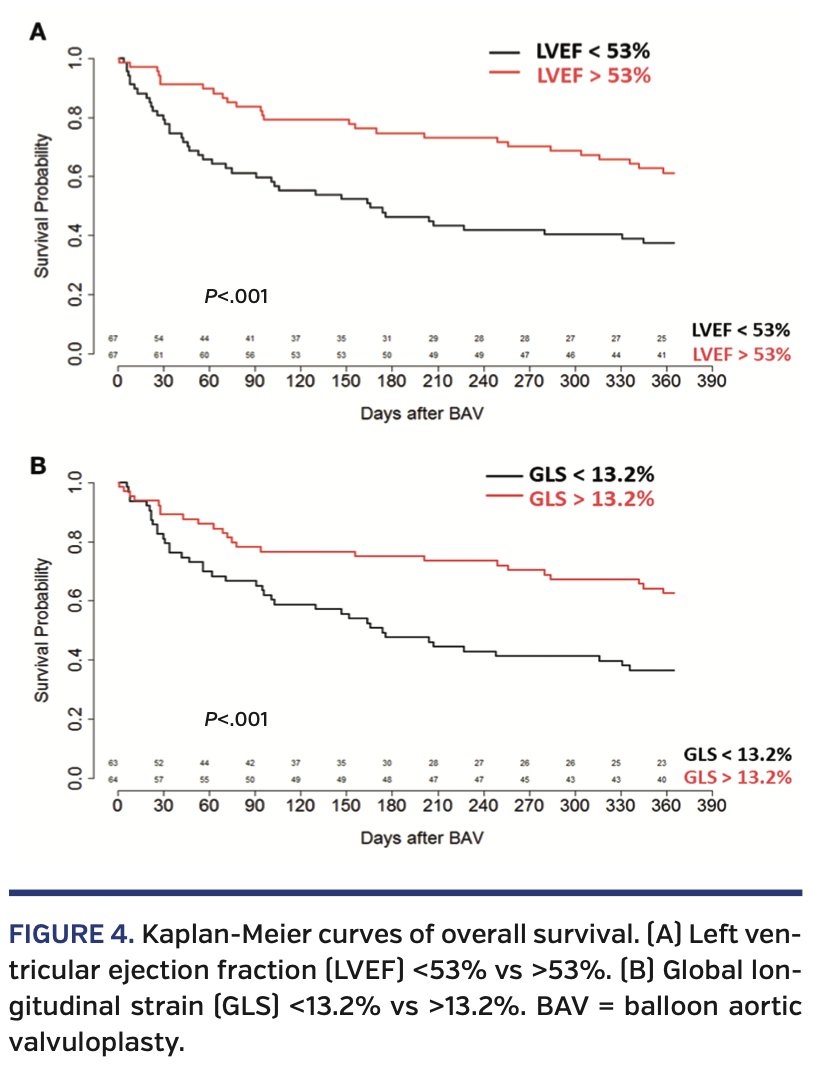
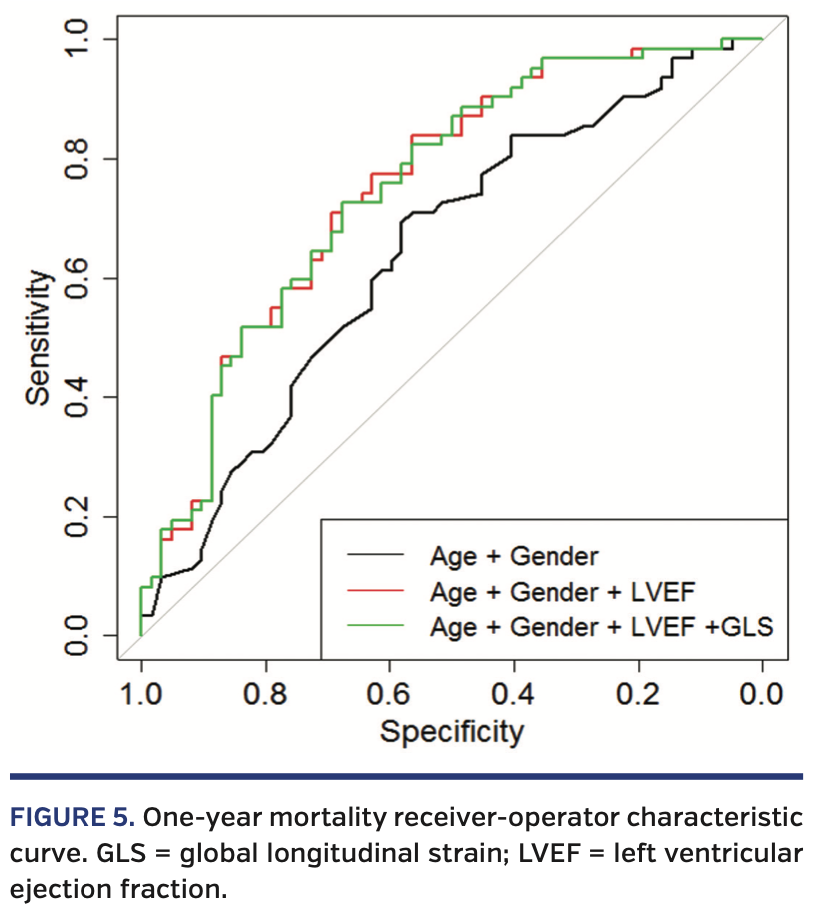
Pre-BAV echocardiographic predictors of mortality. At 1 year, patients with a pre-BAV LVEF less than the median of 53% had a higher mortality rate (P<.001) (Figure 4A), as did patients with an initial LV-GLS less than the median -13.3% (P<.001) (Figure 4B). While both LVEF and LV-GLS values prior to BAV are prognostic for 1-year mortality, LV-GLS did not add predictive value after adjusting for LVEF (c-index 0.719 to 0.720) (Figure 5). We were unable to stratify LV-GLS within patients with either a lower LVEF or higher LVEF, as there were few patients with high LVEF/low GLS (n = 11) and low LVEF/high GLS (n = 11).
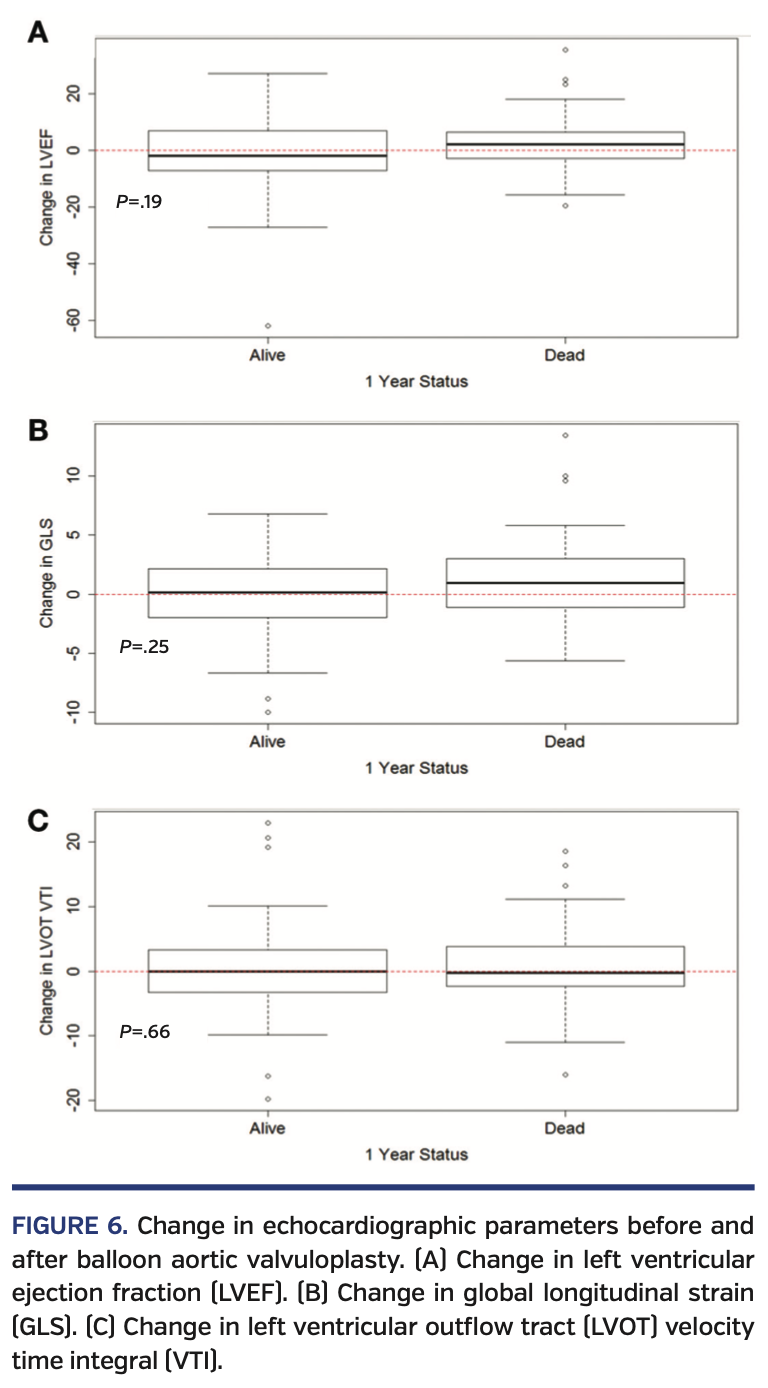
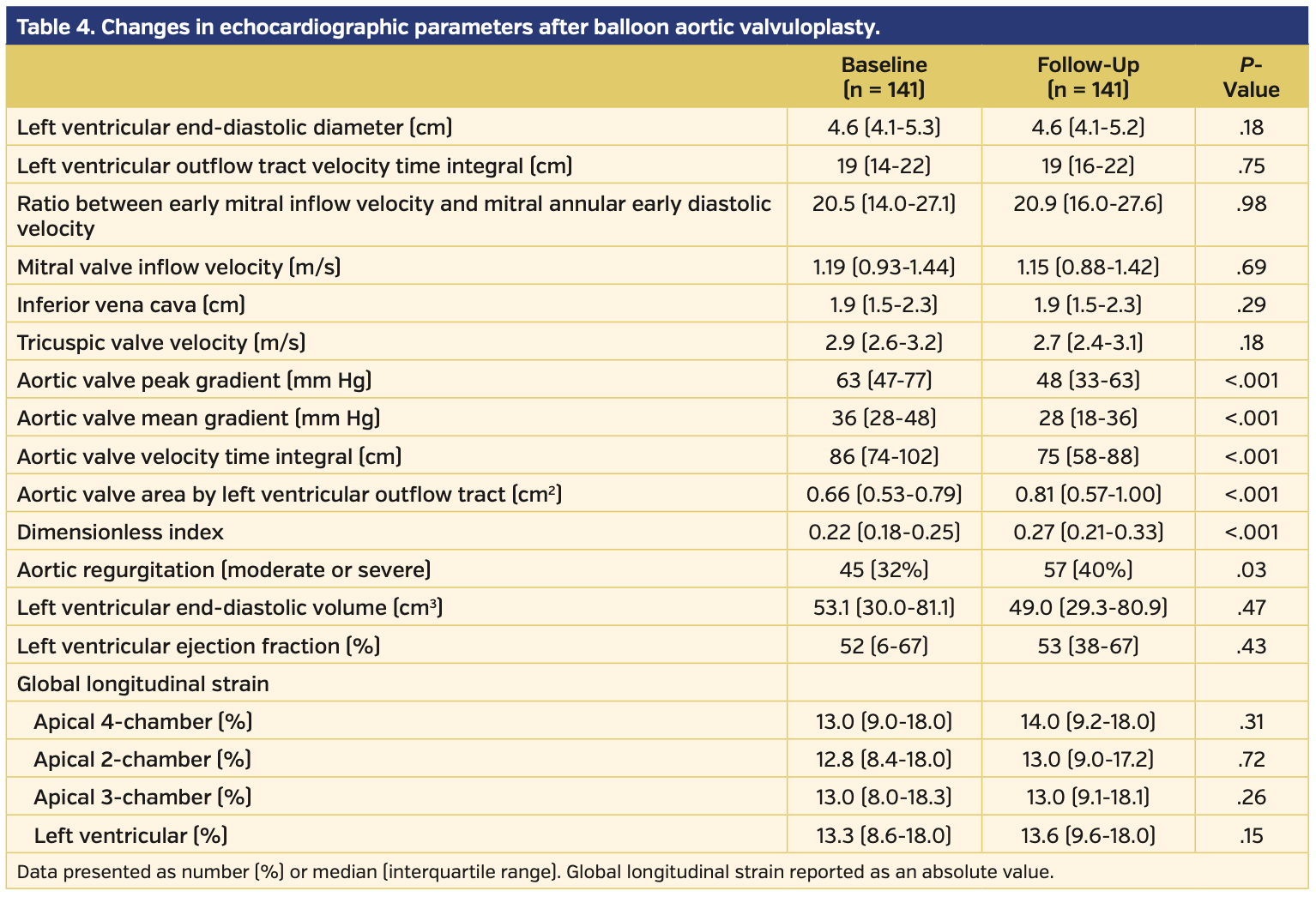
Change in echocardiographic parameters after BAV. After BAV, there was marked improvement in the AV-MG, AV-PG, AVA, and DI, but there was no significant improvement in LV-GLS, LVEF, or left ventricular outflow tract (LVOT) velocity time integral (VTI) (Table 4). There was no significant correlation between the improvement in the AV-MG and the improvement in LVEF or LV-GLS. Between those who died at 1 year and those who survived, there was no difference in LV-GLS, LVEF, or LVOT-VTI (P>.05) (Figure 6).
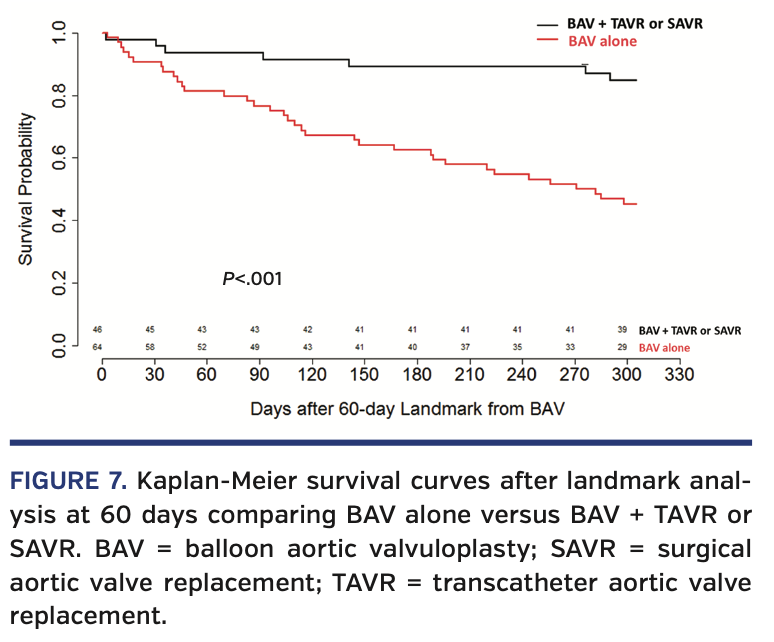
Effect of definitive valve therapy on mortality. There were 47 patients (33%) who eventually had a TAVR/SAVR after BAV. After censoring the first 60 days after BAV, landmark analysis demonstrated that patients who did not undergo definitive valve therapy had a much higher 1-year mortality (hazard ratio, 6.8; P<.001) (Figure 7). Of the patients who underwent TAVR/SAVR, 27 (57%) underwent their definitive valve therapy in the 1-59 days after undergoing a BAV; only 1 patient (3.7%) died prior to the landmark analysis.
Discussion
The major finding of the present study is that among patients with severe, symptomatic AS who undergo a BAV, there is a marked increase in mortality in patients who do not undergo a definitive valve therapy within 1 year. As these patients are at very high risk for mortality, the goal of this study was to predict who will benefit from an invasive BAV procedure and subsequently proceed to definitive valve therapies that are known to prolong survival in patients with symptomatic, severe AS. Predicting these patients is of paramount importance as definitive valve therapies are expensive and should be utilized in those patients most likely to benefit from them.
The largest cohort of patients undergoing BAV was documented in France and assessed 323 high-risk patients with symptomatic, severe AS who underwent BAV as a potential bridge. In this population, there was a high initial procedural success rate with plan to bridge in 74% of the patients; however, only 26% of the patients received definitive therapy, as the remainder were deemed not to be candidates for TAVR or SAVR.2 While this study illustrates the incidence and outcomes of BAV as a bridge procedure, it did not address which patients are likely to go onto definitive valve replacement after BAV.
Tools to identify patients who would then benefit from TAVR or SAVR are lacking. Extensive literature exists that echocardiographic-derived strain is both accurate17 and a stronger marker of LV function and predictor of outcomes when compared with the LVEF.18,19 More specifically, LV strain has been used to assess for acute improvement in LV hemodynamics after TAVR,20,21 but has not been assessed after BAV.
In our analysis, LV-GLS and LVEF offer similar prognostic value for 1-year survival. In clinical practice, LVEF of 53% is considered mildly reduced. However, LV-GLS of 13.2% is considered markedly reduced from recently reported lower limits of normal GLS (17% for men and 18% for women, in absolute values).22 Therefore, LV-GLS may be potentially of higher clinical utility in providing a clear threshold for predicting poor outcomes compared with LVEF. To our knowledge, no prior study has attempted to look at improvement in LV-GLS after a BAV. Prior studies in the pediatric population with congenital AS have either illustrated no improvement in myocardial strain after an aortic valvuloplasty23 or have shown mild improvement, but no return to baseline.24 In the TAVR population, numerous studies have illustrated improvement in strain at different points after the procedure and these have ranged from immediate improvement,21,25 weeks to months20,26,27after the procedure, and 1 year after the procedure.28-30
These results beg the question as to why there is improvement in GLS after a TAVR but not after a BAV. Our hypothesis is that the cohort of patients with severe AS who are unable to undergo definitive valve therapy are sicker, as they are either in cardiogenic shock or have other comorbidities that make them non-optimal candidates. Using LV-GLS as an example, the median LV-GLS in our cohort of 13.3% was lower than the baseline LV-GLS in 2 out of the 3 studies that used LV-GLS in their strain analysis.25,26,29 Thus, the patients in our cohort likely had worse LV systolic function at baseline compared with the patients in the cohorts that underwent TAVR. Furthermore, BAV is not considered to be as durable or as effective of a procedure; an excellent result is only expected to last in terms of months.3-5 Again, using LV-GLS as a comparison, the improvement in LV-GLS (absolute value) after TAVR was on average 2% compared with an improvement of 0.3% after BAV in our cohort.25,29 In this manner, we likely did not observe an improvement in LV-GLS after a BAV, as our patients were sicker compared with those who are candidates for definitive valve therapy, and BAV is clearly an inferior procedure compared with TAVR.
While the mortality in our cohort of patients is markedly high, it is evidence that there is currently a good clinical evaluation of these patients with severe, symptomatic AS who are deemed high risk for definitive valve therapy. Current guidelines mandate that there needs to be at least a 50% survival rate at 1 year in order to have a TAVR program.31 In this high-risk population, definitive valve therapy had a 1-year survival rate of 83%, while the BAV-only cohort had a 1-year survival rate of 31%. This difference in mortality and marked difficulty to discriminate which patients are likely to survive on preprocedure echocardiogram exemplifies the importance of the heart team in the decision-making process, as well as not offering a BAV procedure to all high-risk patients. The results of our study supports the tenet that if a patient survives 30 days post BAV, then they should be re-evaluated by the heart team for a definitive valve therapy unless there are continued contraindications for proceeding.
Study limitations. There are several limitations to the present study. First, this is a post hoc analysis where the results were obtained retrospectively from a single center. Furthermore, the markedly elevated mortality rate created study groups that were not equal in size when trying to compare echocardiographic predictors of mortality. More specifically, the high mortality in this cohort might have hidden any further prognostic value of LV-GLS. Finally, the patients who were clinically deemed to not be candidates for definitive valve therapy after BAV were likely different from those who subsequently underwent TAVR/SAVR; thus, no statement regarding causation should be made due to underlying confounding effects. Our findings can only be hypothesis generating, and further testing is required.
Conclusion
In patients undergoing BAV, there is a very high 1-year mortality rate. In this population, LV-GLS has prognostic value similar to LVEF, but likely has better clinical utility. As patients who undergo TAVR/SAVR have markedly improved mortality, careful consideration should be given to advance definitive valve therapy in carefully selected BAV patients.
From the Department of Medicine, Division of Cardiovascular Medicine, Washington University School of Medicine, Saint Louis, Missouri.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Sintek reports consultant honoraria from Edwards LifeSciences and Philips Volcano Corporation. Dr Gorcsan reports grants from EBR systems, V-Wave Ltd, GE, Hitachi, and TomTec. Dr Zajarias reports personal fees from Edwards LifeSciences. The remaining authors report no conflicts of interest regarding the content herein.
Manuscript accepted March 11, 2020.
Address for correspondence: Alan Zajarias, MD, Division of Cardiology, Department of Medicine, Washington University/Barnes Jewish Hospital, 660 S. Euclid Ave, CB 8086, St. Louis, MO 63110. Email: azajarias@wustl.edu
- Cribier A, Savin T, Saoudi N, et al. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: an alternative to valve replacement? Lancet. 1986;1:63-67
- Eltchaninoff H, Durand E, Borz B, et al. Balloon aortic valvuloplasty in the era of transcatheter aortic valve replacement: acute and long-term outcomes. Am Heart J. 2014;167:235-240.
- Safian RD, Warren SE, Berman AD, et al. Improvement in symptoms and left ventricular performance after balloon aortic valvuloplasty in patients with aortic stenosis and depressed left ventricular ejection fraction. Circulation. 1988;78:1181-1191.
- Safian RD, Berman AD, Diver DJ, et al. Balloon aortic valvuloplasty in 170 consecutive patients. N Engl J Med. 1988;319:125-130.
- Lieberman EB, Bashore TM, Hermiller JB, et al. Balloon aortic valvuloplasty in adults: failure of procedure to improve long-term survival. J Am Coll Cardiol. 1995;26:1522-1528.
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-1607.
- Leon MB, Smith CR, Mack M, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609-1620.
- Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695-1705.
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159-e1195.
- Gorlin R, Gorlin SG. Hydraulic formula for calculation of the area of the stenotic mitral valve, other cardiac valves, and central circulatory shunts. Am Heart J. 1951;41:1-29.
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1-39.e14.
- Park JJ, Park JB, Park JH, Cho GY. Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol. 2018;71:1947-1957.
- Curb JD, Ford CE, Pressel S, Palmer M, Babcock C, Hawkins CM. Ascertainment of vital status through the National Death Index and the Social Security Administration. Am J Epidemiol. 1985;121:754-766.
- Hanna DB, Pfeiffer MR, Sackoff JE, et al. Comparing the National Death Index and the Social Security Administration’s Death Master File to ascertain death in HIV surveillance. Public Health Rep. 2009;124:850-860.
- Rangel C, Shu RG, Lazar LD, Vittinghoff E, Hsue PY, Marcus GM. β-blockers for chest pain associated with recent cocaine use. Arch Intern Med. 2010;170:874-879.
- DeFina LF, Radford NB, Barlow CE, et al. Association of all-cause and cardiovascular mortality with high levels of physical activity and concurrent coronary artery calcification. JAMA Cardiol. Published online 2019;4:174-181.
- Edvardsen T, Gerber BL, Garot J, et al. Quantitative assessment of intrinsic regional myocardial deformation by Doppler strain rate echocardiography in humans: validation against three-dimensional tagged magnetic resonance imaging. Circulation. 2002;106:50-56.
- Kempny A, Diller GP, Kaleschke G, et al. Longitudinal left ventricular 2D strain is superior to ejection fraction in predicting myocardial recovery and symptomatic improvement after aortic valve implantation. Int J Cardiol. 2013;167:2239-2243.
- Nagata Y, Takeuchi M, Wu VC, et, al. Prognostic value of LV deformation parameters using 2D and 3D speckle-tracking echocardiography in asymptomatic patients with severe aortic stenosis and preserved LV ejection fraction. JACC Cardiovasc Imaging. 2015;8:235-245.
- Kim HJ, Lee SP, Park CS, et al. Different responses of the myocardial contractility by layer following acute pressure unloading in severe aortic stenosis patients. Int J Cardiovasc Imaging. 2016;32:247-259.
- Swan A, Prakash R, Chew DP, et al. Instantaneous decrease in left ventricular afterload during transcatheter aortic valve implantation results in immediate changes in left ventricular strain. Echocardiography. 2016;33:742-748.
- Asch FM, Miyoshi T, Addetia K, et al. Similarities and differences in left ventricular size and function among races and nationalities: results of the World Alliance Societies of Echocardiography Normal Values Study. J Am Soc Echocardiogr. 2019;32:1396-1406.e2.
- Friedberg MK, Wu S, Slorach C. Left-right ventricular interactions in pediatric aortic stenosis: right ventricular myocardial strain before and after aortic valvuloplasty. J Am Soc Echocardiogr. 2013;26:390-397.
- Marcus KA, de Korte CL, Feuth T, et al. Persistent reduction in left ventricular strain using two-dimensional speckle-tracking echocardiography after balloon valvuloplasty in children with congenital valvular aortic stenosis. J Am Soc Echocardiogr. 2012;25:473-485.
- Schattke S, Baldenhofer G, Prauka, et al. Acute regional improvement of myocardial function after interventional transfemoral aortic valve replacement in aortic stenosis: a speckle tracking echocardiography study. Cardiovasc Ultrasound. 2012;10:15.
- D’Ascenzi F, Cameli M, Iadanza A, et al. Improvement of left ventricular longitudinal systolic function after transcatheter aortic valve implantation: a speckle-tracking prospective study. Int J Cardiovasc Imaging. 2013;29:1007-1015. Epub 2012 Dec 28.
- Delgado M, Ruiz M, Mesa D, et al. Early improvement of the regional and global ventricle function estimated by two-dimensional speckle tracking echocardiography after percutaneous aortic valve implantation speckle tracking after CoreValve implantation. Echocardiography. 2013;30:37-44. Epub 2012 Sep 18.
- Bochenek T, Kusz B, Mizia M, et al. Echocardiographic evaluation of myocardial strain in patients after transcatheter aortic valve implantation. Postepy Kardiol Interwencyjnej. 2015;11:95-99. Epub 2015 Jun 22.
- Spethmann S, Baldenhofer G, Dreger H, et al. Recovery of left ventricular and left atrial mechanics in various entities of aortic stenosis 12 months after TAVI. Eur Heart J Cardiovasc Imaging. 2014;15:389-398. Epub 2013 Sep 12.
- Løgstrup BB, Andersen HR, Thuesen L, et al. Left ventricular global systolic longitudinal deformation and prognosis 1 year after femoral and apical transcatheter aortic valve implantation. J Am Soc Echocardiogr. 2013;26:246-254. Epub 2013 Jan 8.
- Otto CM, Kumbhani DJ, Alexander KP, et al. 2017 ACC expert consensus decision pathway for transcatheter aortic valve replacement in the management of adults with aortic stenosis: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2017;69:1313-1346. Epub 2017 Jan 4.