Carotid Artery Thrombosis Treated With Catheter Intervention Using Proximal Occlusion and Flow Reversal
Download a PDF of this article.
ABSTRACT: We present a case of symptomatic carotid artery thrombosis treated with catheter intervention under proximal occlusion and flow reversal embolic protection. Although catheter intervention is contraindicated in carotid artery thrombosis due to the risk of distal embolization, the introduction of proximal occlusion embolic protection devices allow interventionalists to use catheter intervention where it was previously deemed too high a risk. A Gore Flow Reversal device was used in a 57-year-old male with obesity, uncontrolled type I diabetes mellitus, hypertension, hyperlipidemia, and prior stroke who had previously undergone left internal carotid artery revascularization with carotid artery endarterectomy and patch angioplasty. Since balloon disruption and manual aspiration through the balloon sheath of the flow reversal device was unable to remove the thrombosis, an AngioJet 4 Fr RX catheter was used to mechanically remove material via mechanical thrombectomy. As there was still residual stenosis, a stent was placed in the area to decrease the remaining blockage. Follow-up carotid artery duplex scanning showed that the procedure eliminated the carotid occlusion. The embolic protection device and the procedural technical aspects are described herein, as are reports of both clinical and anatomical follow-up. We show that by using a Gore Flow Reversal protection device, we were able to use catheter therapy to treat a carotid thrombosis, which was previously contraindicated for this condition.
J INVASIVE CARDIOL 2013;25(5):E106-E109
Key words: emboli, cerebral, intervention, stent, thrombectomy
___________________________________________
The use of catheter intervention is currently contraindicated in the treatment of carotid artery thrombosis due to the high risk of distal embolization.1 However, the recent introduction of proximal occlusion embolic protection devices (POEPDs) allows interventionalists to provide embolic protection before crossing the lesion in the internal carotid artery with a guidewire.2 As a result, the use of POEPDs might expand the indication for carotid catheter intervention to lesions with a thrombus, or other lesions known to have a high risk for distal embolization.3 In this case report, in support of the use of POEPDs in carotid artery thrombosis, we describe the treatment of a symptomatic carotid artery thrombosis using a POEPD with clinical and anatomic follow-up.
Case Report. The patient was a 57-year-old male with obesity, uncontrolled diabetes mellitus type I, hypertension, hyperlipidemia, and previous stroke, who had previously undergone left internal carotid artery (LICA) revascularization with carotid artery endartectomy (CEA) and patch angioplasty. He was transferred to our center with a simultaneous left hemispheric transient ischemic attack (TIA) and an acute inferolateral ST-elevation myocardial infarction (STEMI).
Computed tomography (CT) imaging of the head taken without contrast showed no acute intracranial bleeding and an old right parietal infarction. Coronary angiography revealed an acute left circumflex (LCX) coronary artery occlusion and a partially occlusive filling defect in the left anterior descending (LAD) coronary artery with severe angiographic stenosis. Next, a primary percutaneous coronary intervention (PPCI) was performed using manual aspiration with an Export aspiration catheter (Medtronic, Inc) in the LCX, resulting in revascularization with no underlying angiographic stenosis that would require stenting. Next, manual catheter aspiration successfully retrieved atherothrombotic material from the LAD and the first diagonal branch. In addition, balloon angioplasty and stenting were also performed in the LAD. Both electrocardiographic changes and symptoms resolved at the end of the procedure.
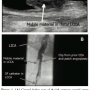 Carotid duplex scanning performed following cardiac intervention revealed a large amount of a mobile, partially occlusive, thrombo-embolic mass in the distal left common carotid artery (CCA) located at the site of a previous CEA and patch angioplasty (Figure 1A). This was treated post procedure using intravenous (IV) heparin for anticoagulation therapy. Carotid artery angiography performed the following day revealed no resolution of the stenosis (Figure 1B). Since the patient met both anatomical (previous ipsilateral CEA) and medical comorbidity (recent STEMI) high-risk criteria for surgical carotid intervention,1 catheter intervention using POEPD was recommended.
Carotid duplex scanning performed following cardiac intervention revealed a large amount of a mobile, partially occlusive, thrombo-embolic mass in the distal left common carotid artery (CCA) located at the site of a previous CEA and patch angioplasty (Figure 1A). This was treated post procedure using intravenous (IV) heparin for anticoagulation therapy. Carotid artery angiography performed the following day revealed no resolution of the stenosis (Figure 1B). Since the patient met both anatomical (previous ipsilateral CEA) and medical comorbidity (recent STEMI) high-risk criteria for surgical carotid intervention,1 catheter intervention using POEPD was recommended.
Embolic protection device. The POEPD of choice for this procedure was a Gore Flow Reversal (GFR) system (W.L. Gore & Associates, Inc). The GFR system has three components: a Gore balloon sheath with a distal balloon to occlude the CCA and to perform as a carotid artery sheath; a Gore wire balloon with a distal balloon to occlude the external carotid artery (ECA); and a Gore external filter to strain the blood flowing from the sidearm of the balloon sheath to a 6 Fr venous access. The balloon sheath is first advanced from an arterial access to the CCA, then the balloon wire is inserted through the balloon sheath to the ipsilateral ECA, and the sidearm of the balloon sheath is attached to a 6 Fr venous access through the Gore external filter, creating an arteriovenous (AV) fistula. Inflating the balloon at the tip of the balloon sheath stops blood flow in the CCA, while inflating the balloon at the tip of the balloon wire stops blood flow in the ECA and prevents retrograde flow from the ECA to the ICA and prevents embolization through the usual distal ECA to ICA collaterals. Occluding the CCA and the ipsilateral ECA in addition to the established AV fistula will result in reversal of blood flow in the ICA. A manifold can then be attached via a three-way stopcock between the balloon sheath sidearm and the Gore external filter and be used for both active manual aspirations and contrast injections. After ensuring flow reversal in the ICA, the lesion is crossed with any guidewire of choice before proceeding with balloon angioplasty, stent deployment, and post dilation. Manual aspiration should be performed during and/or after each step. At the end of the procedure, the ECA balloon is deflated and removed, while forward flow in the CCA is resumed by deflating the CCA balloon while applying manual active aspiration.
The AngioJet catheter (MEDRAD, Inc) mechanical thrombectomy device uses the Venturi-Bernoulli effect of a jet of saline at the tip of the catheter to break up and aspirate thrombi from peripheral and coronary blood vessels; however, the AngioJet catheter has never been evaluated for use in the carotid arteries.
Procedure. Arterial access was obtained using a 9 Fr sheath inserted in the right common femoral artery, while venous access was obtained using a 6 Fr sheath in the left common femoral vein. Anticoagulation and platelet inhibition were achieved with intravenous heparin and integrilin. The left CCA was canulated with a 6 Fr JR 4 diagnostic catheter, which was exchanged over an Amplatz super-stiff wire (Boston Scientific) to the balloon sheath. The balloon sheath sidearm was attached to the venous sheath via a Gore external filter, creating an AV fistula.
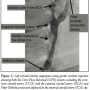
 Because the balloon wire would have to cross the thrombus in the distal CCA, we first stopped the blood flow in the CCA by inflating the balloon at the tip of the Balloon Sheath in the mid CCA segment, and then advanced the Gore balloon wire to the ECA and inflated the balloon (Figure 2). Flow reversal monitoring was performed using a gentle contrast injection (Figure 3). Active manual aspirations were performed following each step throughout the procedure. However, as the superior thyroid artery originates from the carotid bulb, it could not be occluded with the ECA balloon.
Because the balloon wire would have to cross the thrombus in the distal CCA, we first stopped the blood flow in the CCA by inflating the balloon at the tip of the Balloon Sheath in the mid CCA segment, and then advanced the Gore balloon wire to the ECA and inflated the balloon (Figure 2). Flow reversal monitoring was performed using a gentle contrast injection (Figure 3). Active manual aspirations were performed following each step throughout the procedure. However, as the superior thyroid artery originates from the carotid bulb, it could not be occluded with the ECA balloon.
 Because a non-occluded superior thyroid branch could provide collateral circulation and allow antegrade flow to the brain, we decided to use a filter distal EPD simultaneously with the GFR system for added embolic protection. A 7 mm AccuNet filter EPD (Abbott Vascular) was prepared and used to cross the stenosis, and then the basket was deployed in the ICA distal to the carotid bifurcation. Manual aspirations using the balloon sheath did not remove the thromboembolic material in the distal CCA, nor did multiple aspirations following mechanical disruption with balloon angioplasty and snare wires.
Because a non-occluded superior thyroid branch could provide collateral circulation and allow antegrade flow to the brain, we decided to use a filter distal EPD simultaneously with the GFR system for added embolic protection. A 7 mm AccuNet filter EPD (Abbott Vascular) was prepared and used to cross the stenosis, and then the basket was deployed in the ICA distal to the carotid bifurcation. Manual aspirations using the balloon sheath did not remove the thromboembolic material in the distal CCA, nor did multiple aspirations following mechanical disruption with balloon angioplasty and snare wires.
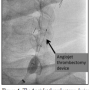 In order to remove the mass, an AngioJet 4 Fr RX catheter was used for mechanical thrombectomy (Figure 4). The AngioJet partially decreased the mass size, but a significant angiographic residual stenosis persisted. A tapered 10-8 × 40 mm, closed-cell, self-expanding Xact stent (Abbott Vascular) was then deployed, and postdilated with 5.5 × 20 mm Viatrac balloon (Abbott Vascular). Stenting resulted in complete resolution of the angiographic stenosis (Figure 5).
In order to remove the mass, an AngioJet 4 Fr RX catheter was used for mechanical thrombectomy (Figure 4). The AngioJet partially decreased the mass size, but a significant angiographic residual stenosis persisted. A tapered 10-8 × 40 mm, closed-cell, self-expanding Xact stent (Abbott Vascular) was then deployed, and postdilated with 5.5 × 20 mm Viatrac balloon (Abbott Vascular). Stenting resulted in complete resolution of the angiographic stenosis (Figure 5).
 The filter EPD was then retrieved, and the balloon wire was deflated and removed. The final step was to allow forward blood flow in the CCA to resume by deflating the balloon at the tip of the balloon sheath. The final cerebral angiogram did not show distal embolization.
The filter EPD was then retrieved, and the balloon wire was deflated and removed. The final step was to allow forward blood flow in the CCA to resume by deflating the balloon at the tip of the balloon sheath. The final cerebral angiogram did not show distal embolization.
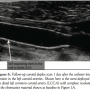
There were no new neurologic changes the following day and a carotid duplex scan showed no residual thrombus (Figure 6).
 However, the patient suffered a sudden cardiac death during the same hospital stay. The autopsy showed no new cerebral infarction, and a 0.8 cm left parietal lobe hemorrhagic transformation of the presenting TIA. A postmortem cardiac examination showed large recent myocardial infarct involving the basilar left anteroseptal myocardium and a large area of the posterolateral myocardium.
However, the patient suffered a sudden cardiac death during the same hospital stay. The autopsy showed no new cerebral infarction, and a 0.8 cm left parietal lobe hemorrhagic transformation of the presenting TIA. A postmortem cardiac examination showed large recent myocardial infarct involving the basilar left anteroseptal myocardium and a large area of the posterolateral myocardium.
Discussion. This patient provided a rare opportunity to anatomically examine the outcome of a high-risk carotid catheter intervention using a POEPD in combination with a filter distal EPD. The autopsy revealed that our embolic protection strategy prevented embolic complications despite mechanical disruption of the thromboembolic material in the distal CCA. Although there are no randomized clinical trials to support this strategy, the use of embolic protection during carotid artery stenting (CAS) is considered the gold standard.1
Mechanical thrombectomy has been used in the coronary arteries and the peripheral blood vessels to remove thrombo-embolic material, but it has not been widely used in the Carotid arteries. The AngioJet catheter uses the Venturi-Bernoulli effect of a saline jet at the tip of the catheter to break and aspirate the thrombi in a blood vessel, but complete prevention of distal embolization has not been demonstrated during mechanical thrombectomy using the AngioJet catheter. The use of the POEPD with flow reversal prevented distal embolization even during significant thrombi disruption induced by the AngioJet use in this patient.
EPDs designed for catheter intervention in the carotid arteries are proximal occlusion embolic protection devices like the GFR system and the Mo.Ma device (Invatec), and distal embolic protection devices (DEPD) like filters and balloon occlusion. These devices differ in that the POEPDs provide embolic protection prior to crossing the lesion in the ICA, while DEPDs are deployed in the ICA distally after crossing the lesion.4 Although the Mo.Ma device provides proximal occlusion embolic protection, it could not be used in this case due to it crossing the lesion in the distal CCA before the deployment of the embolic protection mechanism. However, the GFR system allows us to provide partial protection by occluding the CCA before crossing the lesion.
Distal balloon occlusion DEPD is designed to stop blood flow in the ICA during the procedure.4 However, balloon occlusion of the ICA is not tolerated in 10% of patients. Furthermore, occluding the ICA without occluding the ipsilateral ECA will not prevent embolic events via the collateral circulation from the ECA to the ophthalmic artery or following the deflation of the protecting balloon.5 While POEPDs have the same intolerance problem as the distal occlusion EPDs, the use of POEPDs ensures complete aspiration of embolic material without the need to use a dedicated aspiration catheter. Filter DEPDs have gained a wider acceptance than distal balloon occlusion DEPDs because they are better tolerated by the patients and are easier to use. They also allow normal, forward blood flow and contrast injections during the procedure.4 However, it is important to note that these filter devices will allow particles up to the size of the basket openings (usually 70-140 µm) to enter the brain circulation. Furthermore, care must be taken to ensure that the device is well fitted to the arterial wall to prevent potential embolic material from passing around the filter.4
The use of POEPDs has the potential to expand the indications for catheter interventions in the carotid arteries. CAS can be performed in lesions with high risk for embolization like carotid thrombosis, friable mobile plaque, and carotid lesions with string sign. Nikas and colleagues used POEPDs during CAS procedures in 25 patients with angiographic string sign, a well-known anatomical and functional feature for adverse events because of the possible presence of thrombus at the lesion site, and reported no deaths or strokes over 30 days of postoperative observation.3
In this procedure, we used a filter DEPD simultaneously with a POEPD, because the superior thyroid artery could not be occluded. This strategy is a modification of the so-called “seat belt and airbag strategy,” in which a POEPD is deployed first and then a filter DEPD is advanced through the stenosis and deployed, followed by deactivation of the POEPD to allow normal forward blood through the Filter DEPD.6 This strategy can be used in patients who cannot tolerate prolonged occlusion of the CCA. The efficacy of the POEPD used in this study has been examined in a single-center registry in 1300 consecutive symptomatic and asymptomatic patients during CAS, revealing a death and stroke rate over 30 day of 1.38%.7 If these outcomes can be reproduced in a randomized clinical trial, CAS with a POEPD should become the preferred approach to carotid artery revascularization.
References
- Bates ER, Babb JD, Casey DE Jr, et al. Task force on clinical expert consensus documents. ACCF/SCAI/SVMB/SIR/ASITN 2007 clinical expert consensus document on carotid stenting: a report of the American College of Cardiology Foundation task force on clinical expert consensus documents (ACCF/SCAI/SVMB/SIR/ASITN clinical consensus document committee on carotid stenting). J Am Coll Cardiol. 2007;49(1):126-170.
- Parodi JC, Schönholz, C, Parodi FE, Sicard G, Ferreira LM. Initial 200 cases of carotid artery stenting using a reversal-of-flow cerebral protection device. J Cardiovasc Surg (Torino). 2007;48(2):117-124.
- Nikas DN, Ghany, MA, Sorropago G, et al. Carotid artery stenting with proximal cerebral protection for patients with angiographic appearance of string sign. JACC Cardiovasc Interv. 2010;3(3):298-304.
- Henry M, Polydorou A, Henry I, Polydorou AD, Hugel M. Carotid angioplasty and stenting under protection: advantages and drawbacks. Expert Rev Med Devices. 2008;5(5):591-603.
- Wilentz JR, Chati A, Krafft V, Amor M. Retinal embolization during carotid angioplasty and stenting: mechanisms and role of cerebral protection systems. Catheter Cardiovasc Interv. 2002;56(3):320-327.
- Parodi JC, Schönholz C, Ferreria LM, Mendaro E, Ohki T. “Seat belt and air bag” technique for cerebral protection during cardiac stenting. J Endovasc Ther. 2002;9(1):20-24.
- Stabile E, Salemme L, Sorropago G, et al. Proximal endovascular occlusion for carotid artery stenting: results from a prospective registry of 1,300 patients. J Am Coll Cardiol. 2010;55(16):1661-1667.


















