Capture and Repositioning of Third-Generation Migrated Abdominal Endovascular Graft by Bilateral Femoral Wire Externalization and Pulling by “Horse-Riding” Technique Followed by Balloon EVG Stabilization
Abstract: Endovascular aneurysm repair is becoming the treatment of choice for elderly patients bearing abdominal aortic aneurysms with particular anatomical characteristics. Endovascular grafts are usually oversized to achieve sealing and minimize graft migration, the likelihood of which is also reduced by fixation hooks and barbs in the newer generation grafts. Yet, upward migration of the prosthesis, potentially compromising flow to renal and splanchnic vessels, may still occur acutely and requires timely management. We describe a patient with abdominal aortic aneurysm in whom proximal migration of an endovascular graft occurred, leading to renal and mesenteric artery obstruction, which was successfully managed by means of capturing and repositioning the device with a “horse-riding” technique followed by balloon stabilization of the graft to reduce the risk of re-dislodgment during controlateral leg insertion.
J INVASIVE CARDIOL 2012;24(12):685-688
Key words: abdominal aortic aneurysm, endovascular aneurysm repair, endovascular graft, migration
________________________________________________
Endovascular aneurysm repair (EVAR) has become a suitable alternative to open surgical repair of abdominal aortic aneurysms (AAAs), especially in patients at high surgical risk.1 The percutaneous approach has been associated with reductions in hospital stay, blood loss, need for perioperative intensive care, and early complications.2 However, only 50% of patients with AAA can benefit from this treatment, since 0.5-1.0 cm of normal arterial wall “neck” are required, both proximally and distally, for successful endovascular graft (EVG) anchoring and sealing. Moreover, sizing of aortic EVG is a crucial step for successful endovascular treatment of AAAs. Whereas some proximal oversizing is necessary to obtain adequate sealing between the stent-graft and the aortic wall and minimize type I endoleak, it has been inconclusively associated with migration of the EVG, which, if cranially directed, could result in renal artery occlusion or incomplete AAA exclusion.3 Other factors such as neck angulation, shape and presence of mural thrombus, as well as length of the graft, are also implicated in correct device fixation.
Major developments in design have been introduced during more than twelve years of EVG treatment.4 First generation devices relied on the friction provided by radial force and longitudinal rigidity of the stents to maintain proximal aortic neck fixation. Current EVGs have different transmural fixation systems, such as hooks and barbs, to prevent the migration of the devices. Indeed, the rate of migration has been significantly decreased in the long-term follow-up from first generation to current devices. Yet, this complication may still occur acutely and require timely management.
We hereby report a case of intraprocedural proximal migration of the EVG associated with renal and mesenteric artery obstruction, successfully managed by means of capturing and repositioning of the device with a “horse riding” technique.
Case Description
A 75-year-old man, with ongoing smoking habit and arterial hypertension, was admitted to our institution for percutaneous exclusion of an abdominal aortic aneurysm incidentally discovered by his family practitioner following a computed tomography (CT) scan of the abdomen and pelvis for lumbar vertebral disc herniation. The CT scan disclosed a fusiform AAA measuring 6.2 x 5.1 cm originating below the renal arteries and extending to the level of the aortic carrefour. A limited amount of thrombus was present at the level of the AAA without involvement of the proximal landing zone, which measured 0.7 cm in length. CT scan examination demonstrated suitability for EVAR, and the correct EVG type was chosen based upon size and length.
Given the shared indication for EVAR, both common femoral arteries were accessed using angiographic landmarks, taking care to ensure the puncture was at the center of the common femoral arterial wall. A 0.035-inch guidewire (Storq Standard, Cordis) was then introduced in the aorta and 11 cm 6 Fr sheaths (Cordis) positioned at the puncture site, bilaterally. Then, a Prostar (Abbott Vascular) 10 Fr closure device was advanced and the needles deployed in the right common femoral artery, with the strands of sutures left loose extracorporeally, followed by deployment of a 10 Fr sheath (Cordis) to prepare the main body access site. Subsequently, a Proglide VIP 6 Fr closure device (Abbott Vascular) was inserted over the wire after sheath removal, positioned with medial rotation at 30° and then deployed with the strands of prolene sutures left loose extracorporeally and taped down and out of the way with wide steri-strips. A second ProGlide VIP closure device was then deployed at the same site with lateral rotation at 30° and a 10 Fr sheath introduced to prepare the contralateral leg access.
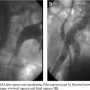
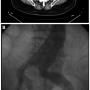 After baseline angiography and positioning of an 0.35 Amplatz Superstiff wire (Boston Scientific) in the aortic arch, an 28 x 140 cm Gore Excluder EVG body (W. L. Gore & Associates) (Figures 1 and 2) was deployed immediately below the lower renal artery. Then, controlateral gate was cannulated by means of a 260 cm 0.035-inch Radifocus wire (Terumo) and a multipurpose (Cordis). The Radifocus wire was exchanged for an Amplatz Superstiff wire and a 18 Fr sheath was carefully advanced to reach the gate region for contralateral deployment of a 18 x 10 cm leg.
After baseline angiography and positioning of an 0.35 Amplatz Superstiff wire (Boston Scientific) in the aortic arch, an 28 x 140 cm Gore Excluder EVG body (W. L. Gore & Associates) (Figures 1 and 2) was deployed immediately below the lower renal artery. Then, controlateral gate was cannulated by means of a 260 cm 0.035-inch Radifocus wire (Terumo) and a multipurpose (Cordis). The Radifocus wire was exchanged for an Amplatz Superstiff wire and a 18 Fr sheath was carefully advanced to reach the gate region for contralateral deployment of a 18 x 10 cm leg.
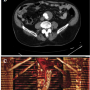
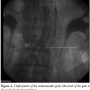 During this maneuver, the dilator of the sheath touched the entrance zone of the gate and dislodged the EVG moving it proximally in the thoraco-abdominal aortic segment causing complete occlusion of both renal arteries and celiac trunk (Figure 3). Despite careful sheath advancement under fluoroscopy guidance, the migration was so rapid that it could not be prevented by sheath retraction. A Radifocus wire was then advanced in the EVG body and by the means of a Simmons 1 catheter (Cordis) the contralateral leg was engaged in a cross-over fashion. A goose-neck 35 mm catheter (Cook) was then advanced from the left femoral artery enabling the capture of the Radifocus wire, whose tip was then
During this maneuver, the dilator of the sheath touched the entrance zone of the gate and dislodged the EVG moving it proximally in the thoraco-abdominal aortic segment causing complete occlusion of both renal arteries and celiac trunk (Figure 3). Despite careful sheath advancement under fluoroscopy guidance, the migration was so rapid that it could not be prevented by sheath retraction. A Radifocus wire was then advanced in the EVG body and by the means of a Simmons 1 catheter (Cordis) the contralateral leg was engaged in a cross-over fashion. A goose-neck 35 mm catheter (Cook) was then advanced from the left femoral artery enabling the capture of the Radifocus wire, whose tip was then 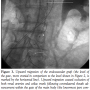 externalized. An over-the-wire 8.0 x 40 mm balloon (Wanda, Boston Scientific) was then inflated at 6 atm in the left common iliac artery to minimize iliac artery injury during manipulations. Then, two torque devices were tightly closed to trap the externalized left and right segments of the Radifocus wire. Under fluoroscopic guidance the devices were then used to forcefully push and tug downwards until the wire was placed in cross-over from the body to the contralateral gate, enabling downward displacement of the EVG and resumption of normal flow in both renal arteries and celiac trunk (Figure 4).
externalized. An over-the-wire 8.0 x 40 mm balloon (Wanda, Boston Scientific) was then inflated at 6 atm in the left common iliac artery to minimize iliac artery injury during manipulations. Then, two torque devices were tightly closed to trap the externalized left and right segments of the Radifocus wire. Under fluoroscopic guidance the devices were then used to forcefully push and tug downwards until the wire was placed in cross-over from the body to the contralateral gate, enabling downward displacement of the EVG and resumption of normal flow in both renal arteries and celiac trunk (Figure 4).
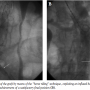 The procedure was then completed by deploying a 20 x 14 cm contralateral leg during inflation of a 34 mm tri-lobated balloon (W. L. Gore) in the EVG body at the level of the proximal stent edge, to stabilize the graft during contralateral sheet gate insertion, with a satisfactory final result (Figure 5). The patient was discharged two days after the procedure, and blood draws did not disclose any evidence of renal damage. Computed tomography demonstrated complete AAA exclusion without any endoleak at up to 6 months follow-up.
The procedure was then completed by deploying a 20 x 14 cm contralateral leg during inflation of a 34 mm tri-lobated balloon (W. L. Gore) in the EVG body at the level of the proximal stent edge, to stabilize the graft during contralateral sheet gate insertion, with a satisfactory final result (Figure 5). The patient was discharged two days after the procedure, and blood draws did not disclose any evidence of renal damage. Computed tomography demonstrated complete AAA exclusion without any endoleak at up to 6 months follow-up.
Discussion
 Graft migration is defined as any movement >10 mm relative to anatomical landmarks, or any migration leading to symptoms or requiring intervention.5 Generally, EVG migration is more commonly caudal than cranial. The former is often associated with type I endoleak, while the latter can result in one, or both, renal arteries occlusion. Whereas migration may occur more often with single-body EVG, such as the Powerlink prosthesis (Endologix), the present clinical vignette shows that it can occur with other devices as well, even with the third generation grafts which present both hooks and barbs to reduce the risk of migration.
Graft migration is defined as any movement >10 mm relative to anatomical landmarks, or any migration leading to symptoms or requiring intervention.5 Generally, EVG migration is more commonly caudal than cranial. The former is often associated with type I endoleak, while the latter can result in one, or both, renal arteries occlusion. Whereas migration may occur more often with single-body EVG, such as the Powerlink prosthesis (Endologix), the present clinical vignette shows that it can occur with other devices as well, even with the third generation grafts which present both hooks and barbs to reduce the risk of migration.
 Both distal and proximal migration usually require surgical conversion given the risk for, respectively, endoleak or major organ ischemia. Thus, migration may often lead to increased morbidity and mortality.4 Whereas, additional deployment of a cuff is feasible in case of caudal migration, a pull-down maneuver such as the “horse-riding” technique hereby reported, can address cranial migrations. Yet, such strategies bear the risk of major complications, including vessel rupture, dissection, and embolization of thrombus. Thus, they should be attempted only by experienced interventionalists, with availability of a wide choice of endovascular materials, and on-site surgical back-up. We found only one other case with a Vanguard graft where a similar technique was utilized to accomplish a correct EVG position. Our case differs from this one by the fact that our graft was of the latest engineering generation which theoretically should offer a much lesser risk of proximal migration once deployed. However, we feel that this occurred because the EVG graft was probably undersized compared to the aortic size and it was not fixed with elastomeric balloon inflation before contralateral leg insertion. On the other hand, the fact that it was not fixed may have facilitated the re-positioning of the graft. In addition, we feel that when it is necessary to deploy an 18 Fr contralateral leg, it should be advanced with extreme care inside the gate always under fluoroscopic guidance to immediately notice EVG movement if it occurs. Moreover, after fixation of the dislodged graft, we utilized a tri-lobate balloon inflation at the proximal neck to stabilize the graft and reduce the risk of new dislodgment during contralateral sheath reinsertion.
Both distal and proximal migration usually require surgical conversion given the risk for, respectively, endoleak or major organ ischemia. Thus, migration may often lead to increased morbidity and mortality.4 Whereas, additional deployment of a cuff is feasible in case of caudal migration, a pull-down maneuver such as the “horse-riding” technique hereby reported, can address cranial migrations. Yet, such strategies bear the risk of major complications, including vessel rupture, dissection, and embolization of thrombus. Thus, they should be attempted only by experienced interventionalists, with availability of a wide choice of endovascular materials, and on-site surgical back-up. We found only one other case with a Vanguard graft where a similar technique was utilized to accomplish a correct EVG position. Our case differs from this one by the fact that our graft was of the latest engineering generation which theoretically should offer a much lesser risk of proximal migration once deployed. However, we feel that this occurred because the EVG graft was probably undersized compared to the aortic size and it was not fixed with elastomeric balloon inflation before contralateral leg insertion. On the other hand, the fact that it was not fixed may have facilitated the re-positioning of the graft. In addition, we feel that when it is necessary to deploy an 18 Fr contralateral leg, it should be advanced with extreme care inside the gate always under fluoroscopic guidance to immediately notice EVG movement if it occurs. Moreover, after fixation of the dislodged graft, we utilized a tri-lobate balloon inflation at the proximal neck to stabilize the graft and reduce the risk of new dislodgment during contralateral sheath reinsertion.
To accomplish the repositioning maneuver, we utilized a balloon to facilitate leg orientation and insertion into the iliac segment. We feel that a gentle “push and tug” technique may decrease the amount of force necessary to dislodge a third generation graft with barbs and hooks from a dislocated position.
In conclusion, the present case describes a patient with AAA in whom proximal migration of an EVG occurred, successfully managed by means of capturing and repositioning of the device, suggests that the “horse-riding” technique may prove a useful adjunct to the armamentarium of endovascular specialists performing EVAR.
References
- Faries PL, Dayal R, Lin S, et al. Endovascular stent graft selection for the treatment of abdominal aortic aneurysms. J Cardovasc Surg. 2005;46(1):9-17.
- Zannetti S, De Rango P, Parlani G, et al. Endovascular abdominal aortic aneurysm repair in highrisk patients: a single centre experience. Eur J Vasc Endovasc Surg. 2001;21(4):334-338.
- van Prehn J, Schlosser FJ, Muhs BE, et al. Oversizing of aortic stent grafts for abdominal aneurysm repair: a systematic review of the benefits and risks. Eur J Vasc Endovasc Surg. 2009;38(1):42-53.
- EVAR Trial Participants. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomized controlled trial. Lancet. 2004;364(9437):843-848.
- Thomas BG, Sanchez LA, Geraghty PJ, et al. A comparative analysis of the outcomes of aortic cuffs and converters for endovascular graft migration. J Vasc Surg 2010;51(6):1373-1380.
__________________________________________
From the 1Department of Cardiology, University of Modena and Reggio Emilia, Modena, Italy, 2Department of Cardiology, Carlo Poma Hospital, Mantova, Italy, and the 3Department of Cardiology, University of Rome Tor Vergata, Rome, Italy.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted February 14, 2012, provisional acceptance given May 1, 2012, final version accepted May 21, 2012.
Address for correspondence: Prof. Giuseppe Sangiorgi, Interventional Cardiology Unit, Department of Cardiology, University of Rome Tor Vergata, Rome, Italy. Email: gsangiorgi@gmail.com.


















