Antiplatelet and Antithrombotic Treatment After Transcatheter Aortic Valve Implantation — Comparison of Regimes
Abstract: Objectives. We compared procedural and follow-up complications of TAVI patients based on the type of antithrombotic treatment used (single-antiplatelet [SAPT] vs dual-antiplatelet [DAPT] vs warfarin). Background. Despite growing operator experience and device development, vascular complications following transcatheter aortic valve implantation (TAVI) remain problematic. Bleeding complications and stroke are two of the main disadvantages compared with surgical aortic valve replacement. Correct choice of antiplatelet or antithrombotic treatment is therefore crucial, but remains empirical. Methods. We analyzed a cohort of 171 patients with symptomatic severe aortic stenosis who underwent TAVI using the CoreValve (Medtronic, Inc) in our center between December 2007 and June 2012. We assessed both procedural, in-hospital, and follow-up outcomes for vascular complications, stroke, myocardial infarction, bleeding complications, and death. Results.Patients were aged 81.6 ± 6.4 years; 47% were male. Treatment regimes were DAPT (34%), SAPT (53%), or warfarin (13%). When analyzing the combined endpoint of all-cause death, acute coronary events, stroke, or bleeding, the outcome was significantly worse in the DAPT group (in-hospital P=.01, 30-day follow-up P=.02). This difference was driven mainly by bleeding complications, with a trend toward higher rates of major bleeding events in the DAPT group vs SAPT group (P=.07 for both in-hospital and 30-day bleeding). The occurrence of major adverse cardiac and cerebrovascular events was statistically similar in all groups.Conclusion. This relatively small series suggests that DAPT does not protect patients from stroke, but may expose them to higher bleeding risk. Further study of this area is warranted.
J INVASIVE CARDIOL 2013;25(10):544-548
Key words: complications, transcatheter aortic valve replacement
_____________________________
Transcatheter aortic valve implantation (TAVI) has become established as a treatment option for patients with symptomatic aortic stenosis. In comparison with surgical aortic valve replacement, TAVI offers superior quality of life with similar mortality rates among patients at very high surgical risk.1 However, thromboembolic complications from TAVI are significant, and stroke in particular is a concern.2 While the immediate procedural risk relates to valvular debris embolization, 50% of strokes develop after the first day and may relate to non-procedural events.1-3 The incidence of cerebrovascular events after TAVI remains raised for≤60 days. This implies that the prothrombotic environment of the bioprosthesis itself may be implicated in distal thromboembolism, and therefore antiplatelet or antithrombotic treatment should play an important role in stroke prevention.4 Various combinations of antithrombotic regimens (single-antiplatelet [SAPT], dual-antiplatelet [DAPT], or warfarin) have been used, but evidence-based guidance remains lacking. The recent ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement did not shed much light on this difficult area.5
It is also important, however, that elderly patients undergoing TAVI are not exposed to unnecessary additional bleeding risk because of relatively aggressive antiplatelet treatments. Vascular complications continue to be seen in ~10% of TAVI cases and some of this bleeding risk may relate to antiplatelet preloading and treatment regimes.1
We therefore examined vascular outcomes with differing antiplatelet/anticoagulant treatment strategies at our center.
Methods
Transcatheter aortic valve implantation has been undertaken at the Sussex Cardiac Centre UK since December 2007. Until May 2010, the antiplatelet policy was for preloading with aspirin 300 mg and clopidogrel 300 mg, followed by 6 months of aspirin 75 mg daily and clopidogrel 75 mg daily. After May 2010, the policy changed to preloading with aspirin 300 mg alone, followed by 6 months of aspirin 75 mg only.
Study population. We analyzed a cohort of 171 patients with symptomatic severe aortic stenosis who underwent TAVI using the CoreValve (Medtronic, Inc) at our center between December 2007 and June 2012. We assessed procedural, in-hospital, and follow-up outcomes for vascular complications, stroke, myocardial infarction, bleeding complications, and death. We compared in-hospital and 30-day outcomes of all 171 patients; we compared 6-month outcomes of 132 patients who had been followed for a minimum of 6 months.
Information on in-hospital outcomes was collected from paper and electronic patient records. Clinical follow-up information was gathered from outpatient clinic visits at 6 weeks and 6 months post procedure. Vital status was tracked via the Office of National Statistics Central Register.
Bleeding score. The Valve Academic Research Consortium (VARC) bleeding definition was used for the purposes of this study. Life-threatening and major bleeding events were recorded. Major bleeding was defined as overt bleeding either associated with a drop in hemoglobin of at least 3 g/dL or requiring transfusion of 2-3 units of whole blood/red blood cells, or causing hospitalization or permanent injury, or requiring surgery. Life-threatening bleeding was defined as fatal bleeding, bleeding in a critical organ, bleeding causing hypovolemic shock or hypotension requiring vasopressors or surgery, or overt bleeding with a drop of hemoglobin of at least 5 g/dL or requiring transfusion of at least 4 units of whole blood/red blood cells.6
Combined endpoints. Two combined endpoints were used in our study. Major adverse cardiac and cerebrovascular event (MACCE) was defined as a combined endpoint of all-cause mortality, acute coronary event or stroke. Net adverse clinical event (NACE) was defined as a combined endpoint of all-cause mortality, acute coronary event, stroke, or major bleeding.
Statistical analysis. Continuous variables are expressed as mean ± standard deviation and were analyzed with the Student’s t-test. Categorical variables are presented as frequency (percentage) and were compared using Pearson’s Chi-Square test or Fisher’s exact test. A two-sided P<.05 was considered to indicate significance. All statistical analyses were performed with SPSS software (SPSS, Inc).
Results
Baseline clinical and procedural characteristics are summarized in Table 1. Patients were aged 81.6 ± 6.4 years, with equal sex distribution. One-fifth were diabetic and more than one-half had a history of coronary artery disease. Patients had severe aortic stenosis with peak gradient 83.8 ± 27.4 mm Hg and aortic valve area (AVA) 0.71 ± 0.22 cm2. Fifty-eight patients (34%) were treated with DAPT, 91 (53%) with SAPT, and 22 (13%) warfarin alone or in combination with antiplatelets. Baseline characteristics did not differ between the three groups, with the exception of atrial fibrillation (AF) prevalence, which was expectedly higher in the warfarin group, and peak aortic gradient, which was higher in the DAPT group.
81.6 ± 6.4 years, with equal sex distribution. One-fifth were diabetic and more than one-half had a history of coronary artery disease. Patients had severe aortic stenosis with peak gradient 83.8 ± 27.4 mm Hg and aortic valve area (AVA) 0.71 ± 0.22 cm2. Fifty-eight patients (34%) were treated with DAPT, 91 (53%) with SAPT, and 22 (13%) warfarin alone or in combination with antiplatelets. Baseline characteristics did not differ between the three groups, with the exception of atrial fibrillation (AF) prevalence, which was expectedly higher in the warfarin group, and peak aortic gradient, which was higher in the DAPT group.
The procedure was successful in 97.7% of patients, with no difference between the three groups. In-hospital mortality was 2.3%, 30-day mortality was 4.7%, and 6-month mortality was 9.1%. Out of the 4 in-hospital deaths, 2 happened within the first year, 1 within the second year, and 1 within the fourth year of the TAVI service. There was no statistically significant difference in mortality between the SAPT and DAPT groups (P=.23, .43, and .52, respectively).
Four patients had a clinical stroke during the first 30 days. These were equally distributed between the SAPT group (n = 2) and the DAPT group (n = 2). One patient on DAPT was rehospitalized due to an acute coronary event within the first 30 days of the TAVI procedure. There were no coronary events in the SAPT group.
between the SAPT group (n = 2) and the DAPT group (n = 2). One patient on DAPT was rehospitalized due to an acute coronary event within the first 30 days of the TAVI procedure. There were no coronary events in the SAPT group.
Bleeding was evaluated using the VARC scoring system. Most of the bleeding events were due to a hemoglobin drop and/or a requirement of transfusion (5 in the SAPT group, 8 in the DAPT group, 1 in the warfarin group). There were only 6 major vascular events, equally distributed in terms of treatment. Three (tamponade [n = 2], femoral artery pseudoaneurysm [n = 1])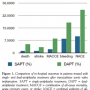 were in the SAPT group and 3 (tamponade, femoral artery rupture, and femoral artery pseudoaneurysm [n = 1 each]) were in the DAPT group. Overall, there was a trend toward higher bleeding rates in the DAPT group, although this difference did not reach statistical significance (P=.069 for both in-hospital and 30-day bleeding).
were in the SAPT group and 3 (tamponade, femoral artery rupture, and femoral artery pseudoaneurysm [n = 1 each]) were in the DAPT group. Overall, there was a trend toward higher bleeding rates in the DAPT group, although this difference did not reach statistical significance (P=.069 for both in-hospital and 30-day bleeding).
The overall occurrence of NACE was significantly higher in the DAPT group in-hospital (P=.01) and at 30 days (P=.017) (Table 2; Figures 1 and 2). Similarly, there was a strong tendency toward adverse outcomes at 6 months; however, this did not reach statistical significance (P=.074) (Table 2; Figure 3).
The warfarin group was very inhomogeneous. Based on the patient’s bleeding risk and the operator’s decision, there were patients on warfarin alone (n = 7), on warfarin in combination with aspirin (n = 11) or clopidogrel (n = 1), and on triple-antithrombotic treatment (n = 3). There were no events in the triple-treatment group, 2 deaths in the warfarin-only group, and 1 bleeding event in the warfarin plus aspirin group. The inhomogeneous character of the warfarin group in combination with the very low number of events made us not draw any conclusions in this group.
operator’s decision, there were patients on warfarin alone (n = 7), on warfarin in combination with aspirin (n = 11) or clopidogrel (n = 1), and on triple-antithrombotic treatment (n = 3). There were no events in the triple-treatment group, 2 deaths in the warfarin-only group, and 1 bleeding event in the warfarin plus aspirin group. The inhomogeneous character of the warfarin group in combination with the very low number of events made us not draw any conclusions in this group.
Discussion
Insecurities regarding the thrombogenicity of bioprosthetic valves and ideal therapeutic regimen are not new in the field of valvular heart disease. After decades of surgical valve replacement, there are still inconsistencies in the recommendations of different professional bodies on ideal thromboembolism prophylaxis.7,8 In the earlier days of experience with bioprosthetic valves, the fear of thrombotic complications translated into general recommendation of 3-month postprocedural warfarin treatment. There is, however, growing evidence that the thrombogenicity of bioprosthetic valves is lower than previously thought and that aggressive antithrombotic treatment might not be necessary.9,10
regimen are not new in the field of valvular heart disease. After decades of surgical valve replacement, there are still inconsistencies in the recommendations of different professional bodies on ideal thromboembolism prophylaxis.7,8 In the earlier days of experience with bioprosthetic valves, the fear of thrombotic complications translated into general recommendation of 3-month postprocedural warfarin treatment. There is, however, growing evidence that the thrombogenicity of bioprosthetic valves is lower than previously thought and that aggressive antithrombotic treatment might not be necessary.9,10
The evidence is even more elusive in the case of TAVI. Earlier pathological study showed thrombus formation and fibrin aggregation on the prosthesis within the first days after implantation.11 Theoretically, thrombus formation may be triggered by a combination of the lack of antithrombogenic properties of native valve endothelium, potential thrombogenicity of fissured, denuded native valve leaflets compressed behind the prosthetic valve, their
interference with complete stent apposition, giving rise to turbulent flow, or stasis behind the valve sealing cuff. Pathological studies have confirmed incomplete neointima formation in the high-velocity flow areas, or areas not in contact with the aortic wall.11 Use of DAPT to abolish the prothrombotic risk early after TAVI was extrapolated from the field of coronary intervention, supposedly taking into account the above-mentioned pathophysiological mechanisms; however, it has never been tested in a large randomized trial. A small randomized study with 79 patients by Ussia et al did not show any difference in outcomes when compared to use of aspirin alone versus DAPT for 3 months after TAVI.12
Similarly, our study did not show significant benefit of adding clopidogrel to prevent major cardiovascular events, and importantly, although not reaching statistical significance, it
showed a strong tendency toward increased bleeding complication rates when compared to treatment with aspirin alone. This is consistent with reports from SAT-TAVI, a single-center randomized trial with 142 patients, which showed similar mortality rates but a trend toward major and minor bleeding reductions with aspirin alone.13 In addition, our study is the first one to show statistically significant superior outcomes of SAPT versus DAPT in terms of NACE.
Bleeding is a frequent complication after TAVI; different studies report major bleeding in up to 20% of patients.14,15 The adverse prognostic impact of bleeding and its effect on long-term mortality is well known in the context of acute coronary syndromes.16 Although not so extensively studied, there have been reports suggesting that major bleeding and use of transfusions have an equally detrimental effect on the outcome in the context of TAVI.14 Possible pathophysiological mechanisms explaining this effect may include hypovolemia, hypotension, impaired oxygen-carrying capacity, suboptimal organ perfusion, and resulting systemic inflammatory response syndrome. This may have been reflected in our study, where we showed a statistically significant difference in NACE in favor of SAPT (P=.01 for in-hospital and P=.017 for 30-day follow-up). This difference did not reach statistical significance at 6 months, which can be explained by the smaller size of the analyzed cohort (P=.08).
The results of the current and the above-mentioned studies suggest that the increased incidence of thromboembolic events post TAVI is not avoidable by simply intensifying the antithrombotic treatment. The possible explanation is that mechanisms other than prosthesis-induced prothrombotic environment play a role in their pathogenesis. More importantly, intensification of the antithrombotic treatment may have detrimental effect on the outcome of the patients, driven mainly by increasing the risk of bleeding complications.
Although small and non-randomized, our study suggests that in the very specific context of percutaneous aortic valve implantation, aspirin alone may provide sufficient protection against thromboembolic complications, while avoiding increased risk of bleeding.
Study limitations. Our study has design limitations. It is a non-randomized and retrospective study, involving data from a single center. Because of its non-randomized fashion, there could be an element of post hoc alignment of endpoints with results, but we did not observe this. The number of patients and events is relatively small, resulting in small absolute differences between the compared groups. The learning curve of the operators occurred chiefly during the DAPT phase of the study and therefore may itself have had an impact on outcomes independent of the antiplatelet treatment. For these reasons, our findings should be considered exploratory, potentially reinforcing the findings of other studies while awaiting results from larger multicenter randomized trials, and at this stage should not encourage change in practice.
Conclusion
For TAVI procedures, aspirin monotherapy may provide sufficient protection against thromboembolic complications, while avoiding increased risk of bleeding.
References
- Smith C, Leon M, Mack M, et al. Transcatheter versus surgical aortic valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187-2198.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med.2010;363(17):1597-1607.
- Kahlert P, Doettger P, Mori K, et al. Cerebral embolization during transcatheter aortic valve implantation: a transcranial Doppler study. Circulation. 2012;126(10):1245-1255.
- Tay EL, Gurvitch R, Wijesinghe N, et al. A high-risk period for cerebrovascular events exists after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2011;4(12):1290-1297.
- Holmes DR Jr, Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59(13):1200-1254.
- Steg PG, Huber K, Andreotti F, et al. Bleeding in acute coronary syndromes and percutaneous coronary interventions: position paper by the working group on thrombosis of the European Society of Cardiology. Eur Heart J. 2011;32(15):1854-1864.
- Butchart EG, Gohlke-Bärwolf C, Antunes MJ, et al; Working groups on valvular heart disease, thrombosis, and cardiac rehabilitation and exercise physiology, European Society of Cardiology. Recommendations for the management of patients after heart valve surgery. Eur Heart J. 2005;26(22):2463-2471.
- Bonow RO, Carabello BA, Chatterjee K, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52(13):e1-e142.
- Gherli T, Colli A, Fragnito C, et al. Comparing warfarin with aspirin after biological aortic valve replacement: a prospective study. Circulation. 2004;110(5):496-500.
- Mistiaen W, Van Cauwelaert P, Muylaert P, Sys SU, Harrisson F, Bortier H. Thromboembolic events after aortic valve replacement in elderly patients with a Carpentier-Edwards Perimount pericardial bioprosthesis. J Thorac Cardiovasc Surg. 2004;127(4):1166-1170.
- Noble S, Asgar A, Cartier R, Virmani R, Bonan R. Anatomo-pathological analysis after CoreValve revalving system implantations. EuroIntervention. 2009;5(1):78-85.
- Ussia GP, Scarabelli M, Mulè M, et al. Dual antiplatelet therapy versus aspirin alone in patients undergoing transcatheter aortic valve implantation. Am J Cardiol. 2011;108(12):1772-1776.
- Stabile E, Sorropago G, Pucciarelli A, et al. SAT-TAVI (Single Antiplatelet Therapy for TAVI) study: a randomized study comparing double to single antiplatelet therapy for transcatheter aortic valve implantation. J Am Coll Cardiol. 2011;58(20)Suppl B:B218.
- Tchetche D, Van der Boon RM, Dumonteil N, et al. Adverse impact of bleeding and transfusion on the outcome post-transcatheter aortic valve implantation: insights from the Pooled-RotterdAm-Milano-Toulouse In Collaboration Plus (PRAGMATIC Plus) initiative. Am Heart J. 2012;164(3):402-409.
- Halliday BP, Dworakowski R, Brickham B, Wendler O, MacCarthy P. Usefulness of periprocedural bleeding to predict outcome after transcatheter aortic valve implantation. Am J Cardiol. 2012;109(5):724-728.
- Mehta SR, Granger CB, Eikelboom JW, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: results from the OASIS-5 trial. J Am Coll Cardiol. 2007;50(18):1742-1751.
________________________
From the Sussex Cardiac Centre, Brighton and Sussex University Hospitals NHS Trust, Brighton, United Kingdom.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Hildick-Smith discloses that he is a proctor for Medtronic CoreValve. Dr de Belder discloses grants from Medtronic, Abbott Vascular, and Boston Scientific, and travel expenses from Abbott Vascular.
Manuscript submitted April 26, 2013, provisional acceptance given May 24, 2013, final version accepted July 22, 2013.
Address for correspondence: David Hildick-Smith, MD, Sussex Cardiac Centre, Eastern Road, BN2 5BE, Brighton, United Kingdom. Email: david.hildick-smith@bsuh.nhs.uk
















