Afterload Mismatch After MitraClip Implantation: Intraoperative Assessment and Prognostic Implications
Abstract: Aim. To evaluate the acute hemodynamic effects after MitraClip implantation and to identify predictors of afterload mismatch and its prognostic implications. Methods. Acute hemodynamic effects were assessed intraoperatively by right heart catheterization and by transesophageal echocardiography before and after MitraClip implantation in 62 consecutive patients with severe mitral regurgitation (MR) (functional MR, 73.8%; EuroScore 2, 7.1 ± 4.9%; left ventricular ejection fraction [LVEF], 36 ± 15%; New York Heart Association class III/IV, 65%). Afterload mismatch was defined as a >15% decrease in LVEF (acute LV depression) or a >15% increase in LV end-diastolic volume (acute adverse LV remodeling). Patients were followed over a period of 24 months (mean, 18 ± 3 months) with all-cause mortality as the primary endpoint. Results. Successful MitraClip implantation with residual MR ≤2 was achieved in 85% of patients. Acute LV depression was observed in 23% of patients, and acute adverse LV remodeling was observed in 15% of patients. Acute adverse LV remodeling occurred in 40% of patients with EuroScores >12 vs in 10% of patients with EuroScores ≤12 (P=.02). Although acute adverse LV remodeling was well tolerated in the acute phase, it was associated with a higher mortality rate during follow-up (62% vs 26%; log-rank P=.04). In a multivariate model, Euroscore 2, but not afterload mismatch, was the most important prognostic risk factor, with an adjusted hazard ratio of 1.1 (95% confidence interval, 1.0-1.2). Conclusion. Afterload mismatch, as assessed intraoperatively, is not uncommon after MitraClip implantation in patients with impaired LV function and is a risk marker of poor clinical outcome.
Key words: afterload mismatch, MitraClip, mitral regurgitation
Mitral regurgitation (MR) is the second most frequent valvular disease in Europe.1 Chronic severe MR leads to dilation of the left ventricle (LV).2 Previous studies have shown that this LV adverse remodeling is associated with a worse outcome in medically managed patients with severe MR.2,3 Mitral valve repair (surgical or percutaneous) can alter the course of adverse remodeling, with improvement of functional status and LV function, reduction of hospitalization for heart failure, and decreased mortality.4-7 MitraClip is a promising, minimally invasive percutaneous treatment technique for severe symptomatic MR in patients with high surgical risk. Studies have shown non-inferiority of MitraClip vs surgical repair,8,9 despite a higher risk profile in the MitraClip group.10-12
However, previous surgical studies of chronic MR have highlighted the risk of afterload mismatch following mitral valve repair, with a sharp decrease in the left ventricular ejection fraction (LVEF). A significant drop in LVEF reflects unmasking of decreased myocardial contractility by mitral valve replacement, with ejection of the total stroke volume into the high impedance of the aorta.13-15 Few studies have investigated the acute hemodynamic effects of MitraClip therapy, with various results. Siegel et al observed a 4% decline in LVEF,16 whereas Melisurgo et al observed a 26% decrease in LVEF.17 These studies evaluated changes in LVEF shortly before and after the procedure, but not intraoperatively. In addition, these studies did not assess acute LV dilation (acute adverse LV remodeling), which was suggested to be a good marker of afterload mismatch by Essandoh.13 To better understand the immediate effect of mitral valve repair on LV mechanics, intraoperative evaluation of LV dimensions and function is required. Therefore, we aimed to describe the acute hemodynamic effects of MitraClip implantation and to identify predictors of afterload mismatch, as assessed intraoperatively, and its prognostic implications.
Methods
Patient population. The study population comprised 62 patients who underwent percutaneous mitral valve repair with MitraClip because of severe symptomatic mitral valve regurgitation despite optimal medical treatment, from 2011 until 2017 at the University Hospital of Antwerp. All patients were deemed to have too high of a surgical risk based upon a global clinical assessment and heart-team evaluation. The ethics committee of Antwerp University Hospital approved the study protocol, and all patients gave written informed consent. The study is registered with clinicaltrials.gov (NCT02506387).
MitraClip procedure. All procedures were performed under general anesthesia using transesophageal echocardiography (TEE) and fluoroscopic guidance. A comprehensive description of the procedure has been previously published.18,19 Postprocedural pharmacologic management included a 3-month prescription of 75 mg clopidogrel daily in addition to aspirin or an anticoagulant, as well as optimal heart failure treatment consistent with heart failure guidelines.
Procedural success was defined as a non-complicated placement of ≥1 clip coinciding with a per-procedure estimated MR reduction to ≤ grade 2. The use of vasopressors or inotropic agents as well as the total volume input during the procedure were registered.
Hemodynamic evaluation. Hemodynamic evaluation was performed by right heart catheterization, with TEE before, during, and after MitraClip implantation. To avoid intraobserver variability, TEE was performed by a single expert sonographer (BP). Serial LV volumes adjusted for body surface area (BSA) were calculated offline using Simpson’s biplane method. MR severity was graded according to the American Society of Echocardiography guidelines based on a validated multi-integrative method.20 Both qualitative (color flow mapping) and quantitative measurements (proximal isovelocity surface area whenever feasible) were used to grade the MR severity from 0 to 4 (grade 0, no/trace; grade 1, mild; grade 2, moderate; grade 3, moderate-to-severe; and grade 4, severe). Right atrium (RA) pressure, pulmonary artery pressure (PAP), and pulmonary capillary wedge pressure (PCWP) were measured by right heart catheterization before and after MitraClip placement. The cardiac index (CI) was measured by the thermodilution method. Afterload mismatch was assessed in two ways: (1) acute adverse LV remodeling, defined as a >15% increase of the BSA indexes of LV end-diastolic volume (LVEDVi) directly after MitraClip implantation; and (2) acute worsening of LV function, defined as a >15% decrease in LVEF directly after MitraClip implantation. The 15% cut-off value was used based upon data from adverse remodeling in heart failure patients with reduced LVEF.2
Follow-up. All patients were followed for up to 24 months (mean follow-up, 18 ± 3 months). The primary endpoint was defined as all-cause mortality. Secondary endpoints were hospitalization for heart failure and the composite of all-cause mortality, hospitalization for heart failure, and reintervention (time to first major adverse cardiac event [MACE]).
Statistical analysis. Categorical variables were labeled as percentages or counts, and continuous variables were described as mean ± standard deviation. Changes in hemodynamic parameters pre- and post-MitraClip implantation were analyzed with paired Student’s t-tests. Between-group comparisons were made with the Chi-square test for categorical variables and with analysis of variance (ANOVA) for continuous variables. Independent predictors of afterload mismatch were assessed by stepwise logistic regression analysis. Receiver operating characteristic (ROC) curve analysis was used to define the optimal cut-off value of EuroScore 2 to predict afterload mismatch. Cumulative event-free survival estimates were plotted using the Kaplan-Meier technique. Differences between the survival curves of the adverse LV remodeling group vs no adverse LV remodeling group were tested with the log-rank test. The Cox proportional hazards model was applied to identify independent predictors of mortality. The following factors were included in the model: EuroScore 2; LVEDVi; success of MitraClip implantation; afterload mismatch; and MR etiology. A two-tailed P-value <.05 was considered statistically significant. Statistical analyses were performed using MedCalc for Windows, version 15.0 (MedCalc Software).
Results
Patient characteristics. The study population comprised 62 patients (53% male) with a mean age of 73 ± 11 years. Most patients (83%) had severe functional MR and were highly symptomatic (New York Heart Association class >2 in 66% of the patients). The study patients were at high surgical risk, as evidenced by a high logistic EuroScore 2 of 7.1 ± 5, depressed LV function (LVEF, 36.3 ± 15%), and impaired renal function (glomerular filtration rate, 50 ± 23 mL/min/1.73 m2). The patients were treated with maximally tolerated heart failure therapy: 87% were on non-potassium sparing diuretics; 11% were on aldosterone blockers; 79% were on beta-blockers; and only 46% were on renin-angiotensin-aldosterone system inhibition.
Hemodynamic changes during the MitraClip procedure. Successful MitraClip implantation was achieved in 85% of patients. Table 1 summarizes the changes in hemodynamic parameters after MitraClip therapy. There was a significant increase in CI (1.4 ± 0.3 L/min/m² to 1.9 ± 0.4 L/min/m²), transmitral gradient (1.5 ± 0.7 mm Hg to 3.7 ± 2.3 mm Hg), and in PAP (35.9 ± 12.1 mm Hg to 38.6 ± 11.2 mm Hg). There were no significant changes in LVEDVi, LVESVi, LVEF, or PCWP. The median increase in LVEDVi was 0.7% (interquartile range 25th-75th [IQR], 11% decrease - 11% increase), and the median increase in LVEF was 4.1% (IQR, 14% decrease - 14% increase).
In 50 patients (80%), vasopressors were used throughout the general anesthesia to maintain systolic blood pressure >100 mm Hg. In 5 patients (8%), inotropic drugs were started from the beginning of the procedure. Patients received 1398 ± 657 mL of fluid during the procedure.
Predictors of afterload mismatch. Acute adverse LV remodeling was observed in 8 of 55 patients (15%), and acute LV depression was observed in 12 of 53 patients (23%). Only 4% of patients showed both acute adverse LV remodeling and acute LV depression. There was no relationship between the changes in LVEDV and changes in LVEF (P=.60; r²=0.007).
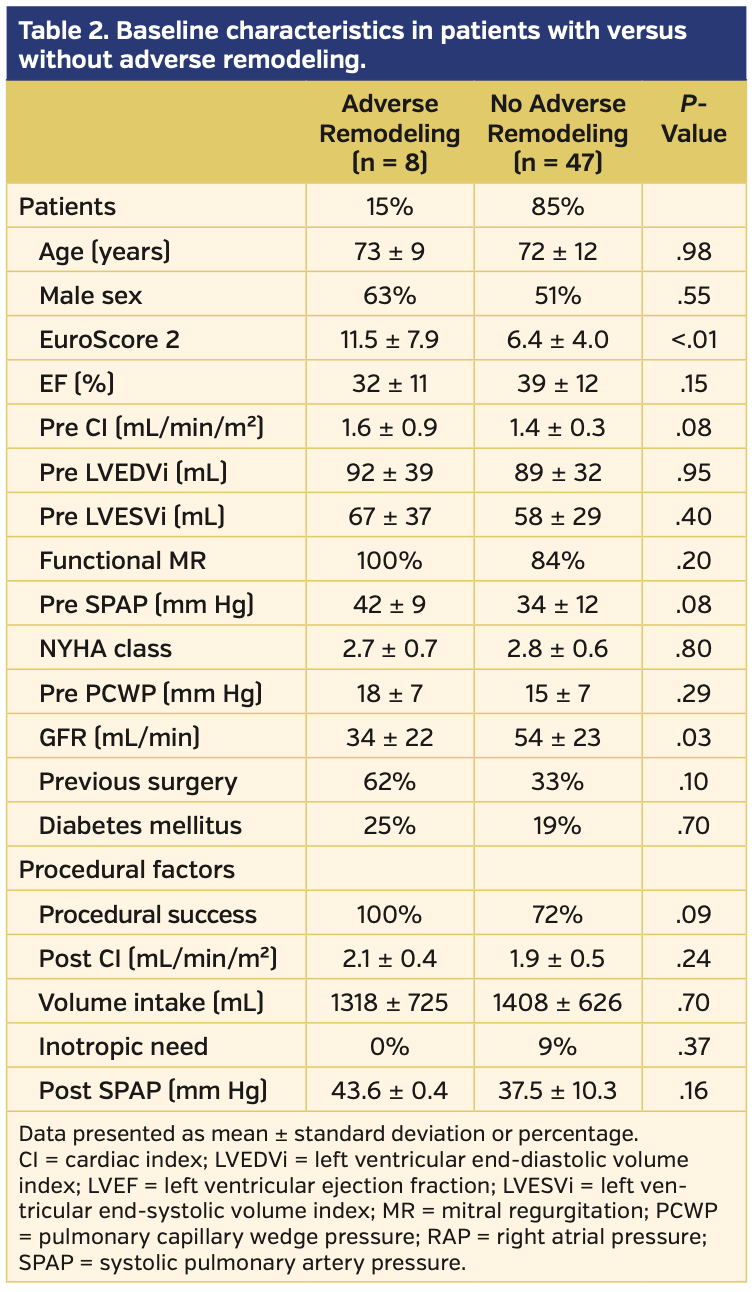
Table 2 compares the baseline characteristics between patients who had adverse LV remodeling and patients who did not have adverse LV remodeling. There was no significant difference in age, LV dimensions, filling pressures, NYHA classification, or etiology of MR. There was a trend of higher systolic pulmonary pressure, more successful MR correction, more previous cardiac surgery and peripheral vascular disease, and more severely depressed LV function in the adverse remodeling group; however, these findings did not reach statistical significance. Only EuroScore 2 (11.5 ±7.9 vs 6.4 ± 4.0; P<.01) and GFR (34.3 ± 22.2 mL/min vs 53.8 ± 23 mL/min; P=.03) were significantly different in patients with adverse remodeling vs patients with no adverse remodeling, respectively.
Multivariate analysis identified EuroScore 2 as the only independent predictor for acute adverse LV remodeling (P=.02; odds ratio, 1.3; 95% confidence interval, 1.03-1.5). Acute adverse LV remodeling occurred in 40% of patients with EuroScore >12 vs in 10% of patients with EuroScore ≤12 (P=.02; area under the ROC curve, 0.74).
Table 3 compares the baseline characteristics between patients with vs without acute LV depression. Multivariate analysis identified only higher filling pressure (PCWP) as an independent predictor of LV depression.
The presence of afterload mismatch did not affect the CI (see post-MitraClip CI values in Tables 2 and 3) and did not require additional inotropic or mechanical support.
Clinical outcome. Patients were followed for up to 24 months, with a mean duration of 18 ± 3 months. In total, there were 20 deaths and 21 hospitalizations for heart failure, as well as 32 patients with at least one MACE (composite of death, reintervention, and hospitalization for heart failure). Patients with acute adverse LV remodeling were more likely to die (75% vs 45% in patients with no acute adverse LV remodeling; log-rank P=.04) (Figure 2) and showed a trend toward a higher MACE rate (88% vs 68% in patients with no acute adverse LV remodeling; log-rank P=.10). There was no association between the presence of acute LV depression and clinical outcome (log-rank P=.80). In a multivariate model, EuroScore 2 was the most important prognostic risk factor, with adjusted hazard ratio of 1.1 (95% confidence interval, 1.0-1.2), whereas markers of afterload mismatch were not independently associated with worse clinical outcomes.
Discussion
Our study demonstrated that the reduction in the regurgitant volume achieved by MitraClip treatment resulted in an acute augmentation in forward cardiac output without affecting LV dimensions or function in most patients. However, in a minority of the patients, afterload mismatch was observed either as acute depression of LVEF (23% of the cases) and/or as acute adverse LV remodeling (15% of the cases). Although this had no clinical impact on short-term outcomes, acute adverse LV remodeling was associated with a worse prognosis.
Previous surgical studies of chronic MR have highlighted the risk of afterload mismatch with the occurrence of acute hemodynamic deterioration, particularly in patients with depressed LV function and/or in patients with dilated LV dimensions and a subsequent limited preload reserve.13,14,21 Those patients sometimes showed severe hemodynamic deterioration requiring prolonged inotropic and/or mechanical support. We and others did not observe such a great hemodynamic impact of afterload mismatch in patients treated with MitraClip. The causes of this prominent postoperative cardiac dysfunction following surgical MR correction have been associated beyond afterload mismatch with myocardial injury and depression of myocardial contractility owing to the use of cardiopulmonary bypass, cardioplegic arrest, and interruption of annular chordal-papillary muscle continuity in non-valve-sparing valve replacement. 22, 23
Previous studies on changes in hemodynamics during the MitraClip procedure showed various results that were probably related to different patient populations. Siegel et al reported hemodynamics in 107 MitraClip patients and found an increase in cardiac output but a decrease in LV end-diastolic volumes 24 hours after implantation. There was a reduction in LVEF (60% vs 56%; P<.001) associated with a reduction in LV end-diastolic volume (172 ± 37 mL vs 158 ± 38 mL; P<.001), while there was no change in LV end-systolic volume (P=NS). The study population mainly comprised patients with degenerative mitral valve regurgitation with good LV function. In these patients, the risk of afterload mismatch was low. In contrast, the risk of afterload mismatch was higher in patients with pre-existing severe LV dysfunction, as demonstrated by Melisurgo et al.17 They showed that in more than one-quarter of patients with severe functional MR, a significant reduction of LVEF (threshold >28%) occurred. In the present study, we identified 23% of patients with significant reduction of LVEF (threshold >15%) post MitraClip. The changes in LVEF seem to be smaller in our study compared with the study by Melisurgo et al.17 This is likely related to the fact that Melisurgo et al included patients with lower LVEF (average, 27%) and excluded patients with post-MitraClip MR grade >2. In our study, 15% of the patients still had an MR grade >2 after intervention, and these patients seem to be less vulnerable to developing afterload mismatches because of the residual low-impedance leak into the left atrium. Additionally, the assessment of afterload mismatch during general anesthesia (as was done in the present study) might have attenuated LV depression, as the systemic afterload will be lower than in conscious patients. The present study also reported acute adverse LV remodeling in 15% of patients; this was mainly observed in patients with severe systolic dysfunction (low LVEF) and diastolic dysfunction (high filling pressure), which is consistent with previous studies. The acute reversal of LV loading conditions post mitral valve repair seems to reveal the true severity of the underlying contractile dysfunction, which is in some patients masked by the presence of a low-impedance leak.
Severe pre-existing LV dysfunction with limited cardiac reserve is most likely also the reason for the observed high mortality rate. The present study highlights that acute adverse remodeling might be a more sensible prognostic marker of poor prognosis than acute worsening of LVEF, which was unrelated to clinical outcome. In the study by Melisurgo et al,17 the observed reduction of LVEF was transient and also not related to clinical outcomes. Other studies have shown persistence of adverse remodeling up to 6 months post Mitra-Clip implantation, which was associated with poor clinical outcomes.7,24 Adverse remodeling has also been associated with higher mortality in other non-valvular interventions, such as post cardiac resynchronization therapy.25 In the present study, acute adverse LV remodeling lost its significance when the EuroScore 2 was incorporated into the multivariate analysis, indicating that it is a risk marker, rather than a risk factor of poor prognosis.
It is unclear whether MitraClip implantation in these patients is still beneficial in addition to medical therapy. Recent randomized trials assessing the additive value of MitraClip in heart failure patients with severe functional MR have shown that some patients (particularly those with end-stage valvular heart disease) will not derive any incremental benefit from MitraClip implantation.26,27 Appropriate patient selection is therefore important.
It would be useful to identify patients who are at increased risk for afterload mismatch post intervention and in whom a valve intervention might be futile. Based upon our findings and the findings of other investigators, standard baseline parameters such as LVEF, LV dimensions, pulmonary pressures, and EuroScore 2 have only a modest value to predict afterload mismatch. Whether assessment of contractile reserve with low-dose dobutamine or with exercise might better predict the development of afterload mismatch post MitraClip implantation is still unknown, but is worthy of investigation when considering the appropriate selection of patients for mitral valve repair.
Study limitations. The study results should be considered in the context of the following limitations. The relatively small number of patients may impede thorough analysis of the different risk factors of afterload mismatch. We could mitigate this limitation, at least partly, by using a more global risk assessment score (as compared with EuroScore 2) in the multivariate analysis. The assessment of the reduction of MR was conducted mainly semiquantitatively, as quantitative analysis of the MR grade after MitraClip implantation is less reliable.
Conclusion
Afterload mismatch, as assessed intraoperatively, is not uncommon after MitraClip implantation, and it seems to be related to severe contractile dysfunction, which is often under-estimated by baseline LVEF. Although it was well tolerated in the acute phase, it should be considered a risk marker of a poor clinical outcome.
From the Departments of 1Cardiology, ²Cardiovascular Surgery, and 3Department of Anesthesiology, Antwerp University Hospital, Edegem, Belgium.
Funding: We thank the Innovation fund of the Antwerp University Hospital for the financial support of the MitraClip device.
ORCID ID Research Number: 0000-0002-6628-9543
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Claeys and Dr Paelinck report honoraria from Abbott. The remaining authors report no conflicts of interest regarding the content herein.
Manuscript submitted August 19, 2019, provisional acceptance given August 26, 2019, final version accepted September 5, 2019.
Address for correspondence: Prof Dr Marc Claeys, MD, PhD, FESC, Department of Cardiology, University of Antwerp Hospital (Edegem), Wilrijkstraat 10 2650 Edegem, Belgium. Email: marc.claeys@uantwerpen.be
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451-2496.
- Nasser R, Van Assche L, Vorlat A, et al. Evolution of functional mitral regurgitation and prognosis in medically managed heart failure patients with reduced ejection fraction. JACC Heart Fail. 2017;5:652-659.
- Agricola E, Ielasi A, Oppizzi M, et al. Long-term prognosis of medically treated patients with functional mitral regurgitation and left ventricular dysfunction. Eur J Heart Fail. 2009;11:581-587.
- Fattouch K, Guccione F, Sampognaro R, et al. POINT: efficacy of adding mitral valve restrictive annuloplasty to coronary artery bypass grafting in patients with moderate ischemic mitral valve regurgitation: a randomized trial. J Thorac Cardiovasc Surg. 2009;138:278-285.
- Foster E, Kwan D, Feldman T, et al. Percutaneous mitral valve repair in the initial EVEREST cohort: evidence of reverse left ventricular remodeling. Circ Cardiovasc Imaging. 2013;6:522-530.
- Kamperidis V, van Wijngaarden SE, van Rosendael PJ, et al. Mitral valve repair for secondary mitral regurgitation in non-ischaemic dilated cardiomyopathy is associated with left ventricular reverse remodelling and increase of forward flow. Eur Heart J Cardiovasc Imaging. 2018;19:208-215.
- Brouwer HJ, Den Heijer MC, Paelinck BP, et al. Left ventricular remodelling patterns after MitraClip implantation in patients with severe mitral valve regurgitation: mechanistic insights and prognostic implications. Eur Heart J Cardiovasc Imaging. 2019;20:307-313.
- Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395-1406.
- Feldman T, Kar S, Elmariah S, et al. Randomized comparison of percutaneous repair and surgery for mitral regurgitation: 5-year results of EVEREST II. J Am Coll Cardiol. 2015;66:2844-2854.
- Philip F, Athappan G, Tuzcu EM, Svensson LG, Kapadia SR. MitraClip for severe symptomatic mitral regurgitation in patients at high surgical risk: a comprehensive systematic review. Catheter Cardiovasc Interv. 2014;84:581-590.
- Glower DD, Kar S, Trento A, et al. Percutaneous mitral valve repair for mitral regurgitation in high-risk patients: results of the EVEREST II study. J Am Coll Cardiol. 2014;64:172-181.
- Nickenig G, Estevez-Loureiro R, Franzen O, et al. Percutaneous mitral valve edge-to-edge repair: in-hospital results and 1-year follow-up of 628 patients of the 2011-2012 Pilot European Sentinel registry. J Am Coll Cardiol. 2014;64:875-884.
- Essandoh MK. Afterload mismatch after MitraClip implantation: the potential impact of pharmacologic support. J Cardiothorac Vasc Anesth. 2017;31:702-706.
- Ross J Jr. Afterload mismatch in aortic and mitral valve disease: implications for surgical therapy. J Am Coll Cardiol. 1985;5:811-826.
- Ashikhmina EA, Schaff HV, Suri RM, Enriquez-Sarano M, Abel MD. Left ventricular remodeling early after correction of mitral regurgitation: maintenance of stroke volume with decreased systolic indexes. J Thorac Cardiovasc Surg. 2010;140:1300-1305.
- Siegel RJ, Biner S, Rafique AM, et al. The acute hemodynamic effects of MitraClip therapy. J Am Coll Cardiol. 2011;57:1658-1665.
- Melisurgo G, Ajello S, Pappalardo F, et al. Afterload mismatch after MitraClip insertion for functional mitral regurgitation. Am J Cardiol. 2014;113:1844-1850.
- Vandendriessche T, Kotrc M, Tijskens M, et al. Percutaneous mitral valve repair in high-risk patients: initial experience with the Mitraclip system in Belgium. Acta Cardiol. 2014;69:265-270.
- Tamburino C, Ussia GP, Maisano F, et al. Percutaneous mitral valve repair with the MitraClip system: acute results from a real world setting. Eur Heart J. 2010;31:1382-1389.
- Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777-802.
- Quintana E, Suri RM, Thalji NM, et al. Left ventricular dysfunction after mitral valve repair — the fallacy of “normal” preoperative myocardial function. J Thorac Cardiovasc Surg. 2014;148:2752-2760.
- David TE, Burns RJ, Bacchus CM, Druck MN. Mitral valve replacement for mitral regurgitation with and without preservation of chordae tendineae. J Thorac Cardiovasc Surg. 1984;88:718-725.
- Abu-Omar Y, Taggart DP. Off-pump coronary artery bypass grafting. Lancet. 2002;360:327-330.
- Adamo M, Godino C, Giannini C, et al. Left ventricular reverse remodelling predicts long-term outcomes in patients with functional mitral regurgitation undergoing MitraClip therapy: results from a multicentre registry. Eur J Heart Fail. 2019;21:196-204.
- Solomon SD, Foster E, Bourgoun M, et al. Effect of cardiac resynchronization therapy on reverse remodeling and relation to outcome: multicenter automatic defibrillator implantation trial: cardiac resynchronization therapy. Circulation. 2010;122:985-992.
- Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379:2307-2318. Epub 2018 Sep 23.
- Obadia JF, Messika-Zeitoun D, Leurent G, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. 2018;379:2297-2306. Epub 2018 Aug 27.