ADVERTISEMENT
Recent and Future Drug Approvals for Important Disease States
Boston—With an influx of blockbuster drugs coming off patent in the next few years, in 2015, a potential $20.9 billion revenue could be reached for first-time generics, according to Christopher Peterson, PharmD, director, emerging therapeutics, Express Scripts, Bloomington, Minnesota, who presented a session about brand and generic drugs in the pipeline at the AMCP meeting.
Top drugs losing patent in 2015 include Abilify® (aripiprazole), Avodart® (dutasteride), Gleevec® (imatinib mesylate), Namenda® (memantine), and Nexium® (esomeprazole). In 2016, Advair® (fluticasone/salmeterol), Benicar® (olmesartan), Crestor® (rosuvastatin), Seroquel XR® (quetiapine fumarate), and Zetia® (ezetimibe) will also lose patent, all opening up significant potential for the generic market.
At the time of the presentation, 30 drugs had been FDA approved in 2014. Of those 30 drugs, 19 were for specialty indications, with an additional 10 more expected by the end of 2014. According to Dr. Peterson, “2010 was the ‘flip-flop’ year.” It was the first year that more specialty medications were approved than traditional drugs. “It has been that way ever since,” said Dr. Peterson.
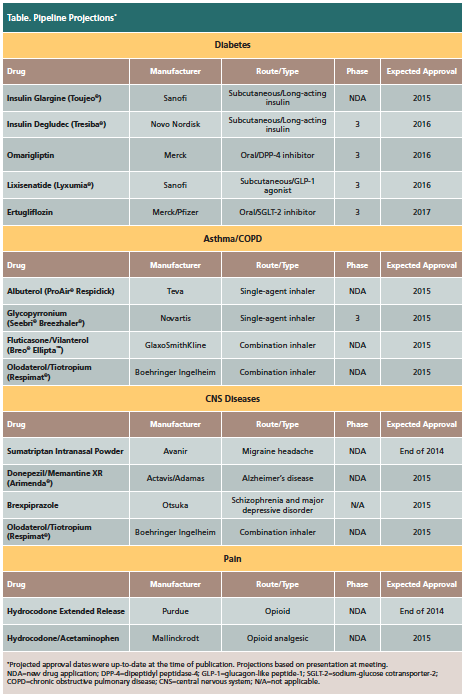
Dr. Peterson discussed drugs that had been approved this year prior to the presentation, while also highlighting projected pipeline drugs with upcoming expected approvals (Table).
Diabetes
The diabetes drug market share comprises $44 billion in worldwide sales, according to Dr. Peterson. A total of 6 diabetes drugs had been approved in 2014 at the time of the presentation, including Farxiga™ (dapagliflozin), Tanzeum™ (albiglutide), Afrezza® (insulin, inhalation powder), Jardiance® (empagliflozin), Invokamet™ (canagliflozin/metformin), and Trulicity™ (dulaglutide). “[Diabetes] has been sheltered from generics,” said Dr. Peterson, though he projected that the next wave of generic diabetes medications would likely begin early in the next decade.
The diabetes pipeline is substantial, and Dr. Peterson said a diabetes biosimilar is likely in the coming years.
Asthma/Chronic Obstructive Pulmonary Disease (COPD)
As the population ages, COPD will become more prevalent. A total of 5 COPD drugs had been approved in 2014 at the time of the presentation, including Asmanex® HFA (mometasone), Incruse® Ellipta™ (umeclidinium), Striverdi® Respimat® (olodaterol), Arnuity™ Ellipta™ (fluticasone furoate), and Spiriva® Respimat® (tiotropium).
Dr. Peterson said generic COPD agents are not expected until 2016, though he projected that Advair Diskus® (fluticasone propionate) will become generic once its patent expires in August 2016. Dr. Peterson also mentioned that there is discussion on whether Singular® (montelukast) will become an over-the-counter (OTC) product in the next few years. In May, however, a committee advised against the OTC status of montelukast due to fears that patients would inappropriately use the drug for asthma, though it is not indicated for its use. The FDA is expected to decide on this before the end of the year.
Central Nervous System (CNS) Diseases
According to Dr. Peterson, approximately 20% of the US population has a CNS disorder. A total of 3 branded CNS drugs had been approved in 2014 at the time of the presentation, including Qudexy™ XR (topiramate extended-release [ER]), Belsomra® (suvorexant), and Contrave® (bupropion/naltrexone). In addition, 3 CNS drugs had gone off patent in 2014 at the time of the presentation, giving an opportunity for generics, including Lodosyn® (carbidopa), Lunesta® (eszopiclone), and Precedex™ (dexmedetomidine).
Pain
Ten percent of the population has moderate to severe pain. A total of 3 branded pain drugs had been approved in 2014 at the time of the presentation, including Xartemis™ XR (oxycodone/acetaminophen), Bunavail™ (buprenorphine/naloxone), and Targiniq™ ER (oxycodone/naloxone ER). In addition, 3 pain drugs had gone off patent in 2014 at the time of the presentation, giving an opportunity for generics, including Avinza® (morphine ER), Exalgo® (hydromorphone ER), and
OxyContin® (oxycodone ER).—Kerri Fitzgerald