ADVERTISEMENT
Impact of Biosimilars on Stakeholders
San Diego—Biologic drugs have become well-established therapeutic options for a variety of diseases. As their use has increased, costs to the US healthcare system have soared in response. In March 2015, the first biosimilar drug fligrastimsndz was approved. The FDA’s biosimilar approval pathway, 351(k), was created through the Biologics Price Competition and Innovation Act of 2009, initiated under the Patient Protection and Affordable Care Act. Additionally, the queue of other biosimilars awaiting approval is growing.
During a satellite symposium at the AMCP meeting, a panel discussed the biosimilars on the horizon and how stakeholders can prepare for the eventual reality of biosimilar competition, to capitalize on potential savings opportunities. The symposium was supported by an educational grant from Hospira, Inc.
Andrew Mulcahy, PhD, MPP, associate policy researcher, The RAND Corporation, opened the session with a discussion on the inception and likely impact of biosimilars in the US healthcare system. Biosimilars are fundamentally different from small-molecule generics. There are more unknowns in biosimilar regulation, litigation, and markets. The focus now from clinical and policy perspectives has been on the short-term concerns and education, not on long-term impacts and potential. The local impact of biosimilars on clinicians, patients, and payers is still significant and important.
“Biosimilars will be extremely important to clinicians,” said Dr. Mulcahy.
He touched on challenges of biosimilars to the healthcare industry such as not knowing the final FDA regulations on regulatory requirements, naming, and interchangeability; and the level of market competition and price discounts. “The single most important question—What will the market look like in 5 years,” he said.
Dr. Mulcahy continued the session by discussing a recent study he coauthored with colleagues on the potential cost savings of biosimilar drugs. For the study, which was published in November 2014 by the RAND Corporation, the authors developed a framework based on economic theory to describe the range of factors that will affect the potential cost savings from biosimilars. The framework identified 4 categories of drivers that together determine the magnitude of cost savings: (1) safety and efficacy compared with other therapeutic options; (2) payment policy and incen- tives for prescribing; (3) acceptability to patients and providers; and (4) competition.
“I think competition is the most important. The number of manufacturers getting involved will impact cost savings,” he said.
Based on the available information and survey of the literature, they calculated a potential direct cost savings of $44.2 billion over 10 years. Key assump- tions factored in to reach this figure included 10% annual biologic sales growth; up to 20% exposure to biosimilars at year 10; 60% biosimilar market share; 35% biosimilar price discount; and different treatment of insulin and growth hormone markets.
The savings will benefit a range of stakeholders. Private and public health insurers will benefit from lower unit prices in the short-term. Patients will benefit immediately through lower cost sharing. In the long- term, these savings should accrue to beneficiaries and taxpayers, according to Dr. Mulcahy. He noted that all cost estimates, including findings from the RAND study have important limitations:
• Heterogeneity across delivery channels
• Payment and delivery innovation
• Nonprice competition from originators or biosimilar manufacturers
• Regulatory and litigation uncertainty
• Indirect health and cost impacts from broader biologic use
Despite the challenges and uncertainty, he concluded that biosimilars will have an important impact on patients, especially those with no or limited coverage of specialty drugs; healthcare system spending; health systems and clinicians adopting these new products; and, in the long-term, ensuring a robust, innovative biologic market.
Implementation of Biosimilars
Biosimilar manufacturing remains a complex process, requiring numerous steps and critical review processes, explained Ali McBride, PharmD, MS, BCPS, BCOP, clinical coordinator, hematology/oncology, University of Arizona Cancer Center.
Formulary management is another important concern for pharmacists and other clinicians to successfully manage the introduction of biosimilars into the health system. The pharmacy and therapeutics (P&T) committee processes will need to develop critical review points of biosimilars for implementation into practice. Unlike generics, initial adoption of biosimilars to health system formularies will be more complex. It also requires substantial education of all clinicians and patients, said Dr. McBride. He listed key questions that will arise for P&T formulary management with regard to biosimilar approvals and implementation.
• What data are being used for approval of the biosimilar?
• Is the clinical efficacy and safety of the biosimilar a concern from the phase 1/3 clinical trial?
• Will payers pay for off-label use of the biosimilar?
• Are side effects similar to the originator product?
“Education continues to be one of the greatest needs for understanding and potentially implementing bio- similars into practice,” he said.
Healthcare professionals, particularly those on P&T committees, will be very influential in driving adoption of biosimilars. However, a lack of awareness and information regarding these agents is one of the most significant problems that hinder the adoption of biosimilars. Dr. McBride cited a study conducted in 2011 at the 16th Annual Conference of the National Comprehensive Care Network that surveyed 277 healthcare professionals including pharmacists (n=38), physicians (n=129), and nurses (n=71). The findings showed that only 13%, 8%, and 12%, respectively, considered themselves “extremely familiar” with the abbreviated regulatory process for biosimilar approval, while 18%, 39%, and 44%, respectively, considered themselves “not at all familiar.”
Payer Perspective
Bill Martin, vice president, pharmaceutical strategies and account management, Accredo Health Group, Inc., concluded the session by discussing the payer perspective. He said a trend to watch is the manage- ment of specialty drugs. He cited results from the 2014 Kaiser Family Foundation and the Health Research & Educational Trust survey. The survey tracked the benefits of 2052 firms, of which 90% offer health benefits and 98% have prescription drug benefits. The findings showed that 80% have a formulary with 3 or more tiers; 4th tier use grew from 14% in 2012 to 20% in 2014; only 10% had a 2-tier plan and 5% had a single tier; and in plans with 3 or more tiers, 49% were subject to coinsurance.
He said that from a payer perspective, interchangeability of biosimilars does matter, citing the following reasons:
-
Copay design, step therapy, and grandfathering are issues that payers can control
-
First biosimilars likely to be payer-driven, market development dependent
-
Biosimilars pipeline will cease to be viable if no success in the United States
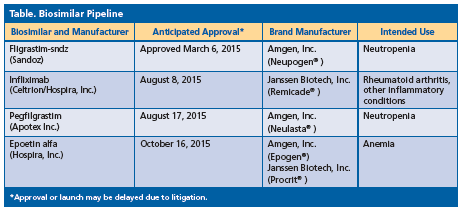
Mr. Martin also reviewed the biosimilars accepted for filing from the FDA (Table). He emphasized, “The first approved [biosimilar will not] necessarily be the first to market.”
In summary, he said that traditional tools such as formularies, tiers, exclusions, and network controls will be important to payers. Furthermore, managing specialty categories is not “off limits” anymore.—Eileen Koutnik-Fotopoulos