Autoimmune Diseases: Rheumatoid Arthritis and Psoriatic Arthritis
 As the health care landscape continues to evolve, managed care professionals are on the frontlines, handling the developing trends that, in turn, have a direct impact on their covered lives. The First Report Managed Care Trends Report serves as a resource for managed care professionals making management decisions addressing the spectrum of disease states that continue to challenge health care providers.
As the health care landscape continues to evolve, managed care professionals are on the frontlines, handling the developing trends that, in turn, have a direct impact on their covered lives. The First Report Managed Care Trends Report serves as a resource for managed care professionals making management decisions addressing the spectrum of disease states that continue to challenge health care providers.
This sixth edition of the Trends Report is a 3-part series on autoimmune diseases, with a focus on 5 autoimmune diseases impacting health care professionals and formulary decision-makers. Autoimmune disease as a category affects 50 million Americans and has been reported to be on the rise in the United States and around the world, “making this poorly understood category of disease a public health crisis at levels comparable to heart disease and cancer.”1
Data for this Trends Report was generated through a comprehensive survey of managed care professionals. Survey questions were developed by the First Report Managed Care editorial staff and vetted by 9 members of the First Report Managed Care Editorial Advisory Board. The types of questions asked in the survey focused on demographics, institutional practices, treatment guidelines for the 5 disease states, compliance and adherence measures, prior authorization criteria, and treatment decisions for some drug classes used in the management of autoimmune disease. Participants were also asked to provide comments on the challenges of treating the 5 autoimmune diseases covered in Trends Report from a managed care perspective.
The survey was distributed via a recognized survey platform and sent to managed care professionals from our email database. A total of 39 managed care professionals completed the survey with 11 partial completions. Data was then compiled based on all of the participants to generate this report.
Part 1 of this 3-part Trends Report focuses on the demographics and institutional practices of the survey participants as well as the findings regarding rheumatoid arthritis (RA) and psoriatic arthritis management.
The complete Trends Report can be downloaded on Managed Health Care Connect at www.managedhealthcareconnect.com/supplements.
Demographics
Institutions and Roles
When asked to best describe the organization for which they work, health maintenance organization and pharmacy benefit manager each received a 22% response from the survey participants. The majority (28.0%) said “other” with written responses including hospital, accountable care organization, specialty pharmacy, and private practice. See Figure 1. The survey participants were asked if their organization has responsibility for commercial insurance plans, Part D insurance plans, and/or Medicare insurance plans—with the option to choose all that apply—and 93.9% said commercial, 73.5% said Medicaid, 71.4% said Part D, and 63.3% said health care exchange (Affordable Care Act) individual plans. See Figure 2. 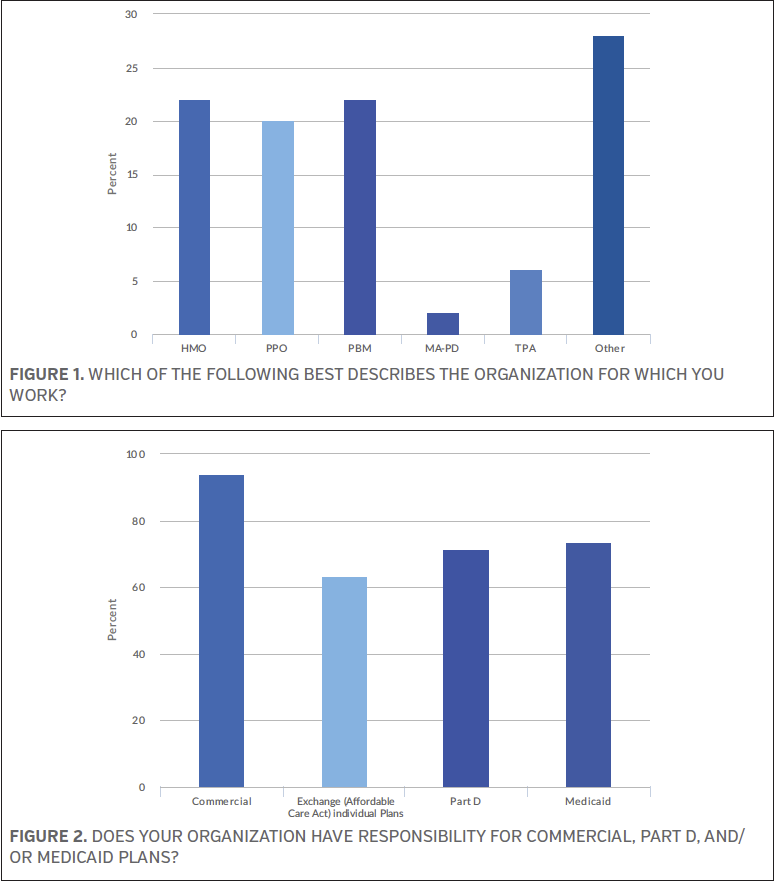
The survey respondents were next asked about the scope of their organization, with 38.0% and 34.0% stating national and regional, respectively; 28.0% said single state. See Figure 3.
When asked how many total lives are covered by their plan, nearly one-quarter of the survey participants (24.5%) said >5,000,000, while 22.4% said ≤100,000. See Figure 4.
The survey respondents were asked to provide their title, and 28.0% said “other,” with responses including physician, clinical pharmacy manager, pharmacy purchaser, and strategy director, among others. Another 24.0% said clinical pharmacist and 22.0% said pharmacy director. See Figure 5 for a complete list of answer options and percentages.
Just over one-quarter of the survey participants (26.0%) said their role regarding formulary inclusion/formulary management reimbursement decisions is best described as voting member of pharmacy and therapeutics (P&T) committee, while 16.0% said they are considered a direct influencer of the P&T committee and another 16.0% said they are an indirect influencer of the P&T committee. See Figure 6.
Institutional Practices
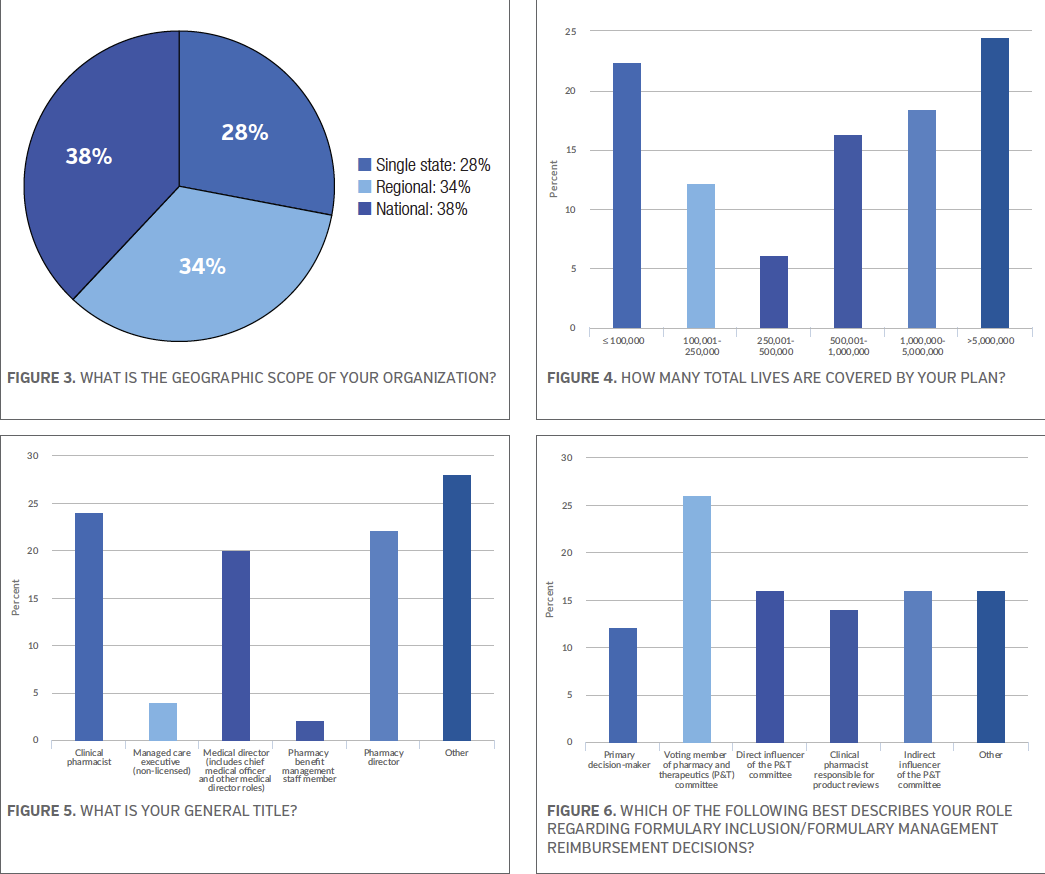
The survey participants were then asked a series of questions about formulary, adherence, and dispensing rates. The majority of the survey respondents (67.3%) said adherence was measured by pharmacy claims data, while 49.0% said medical record review and 36.7% said pharmacy benefit management provided the data. Survey participants were asked to choose all the apply. See Figure 7.
When asked if their primary formulary is open or closed, 40.0% of those who participated in the survey said closed, while 34.0% said open but with selected exclusions, and 20% said open. See Figure 8.
Based on lives covered, the survey participants were asked about how many tiers their common commercial formulary and common Medicare formulary have? According to the results, the majority of the survey respondents (34.0%) said their most common commercial formulary has 3 tiers, while 26.0% said they have 4 tiers. Thirty percent of the survey respondents said their most common Medicare formulary has 3 tiers and 20.0% said 5 tiers. See Figures 9 and 10.
In terms generic dispensing rate, 32.7% said their generic dispensing rate is 85.0%, while 18.4% said they have a 82.5% to 84.9% generic dispensing rate, and another 18.4% said they have a 75.0% to 79.9% generic dispensing rate. See Figure 11.
The final question on demographics asked the survey participants who negotiates pharmacy contracts on behalf of their organization. More than half of those took the survey (57.1%) said internal staff, followed closely by 51.0% who said pharmacy benefit management. See Figure 12.
Rheumatoid Arthritis
RA is an autoimmune disease in which the body’s immune system mistakenly attacks the joints instead of attacking foreign substances likes bacteria and viruses. This misguided reaction creates inflammation that causes the tissue in the joints to thicken, resulting in swelling and pain in and around the joints and possible cartilage damage if untreated. This autoimmune disease most commonly affects the hands, feet, wrists, elbows, knees, and ankles. Nearly 1.5 million people in the United States have RA, of which more than half are women.2 RA most commonly develops in women between ages 30 and 60, while in men it occurs later in life.2 Although having a family member with RA increases the chance of having the disease, the majority of individuals have no family history of the disease.2
Survey Results
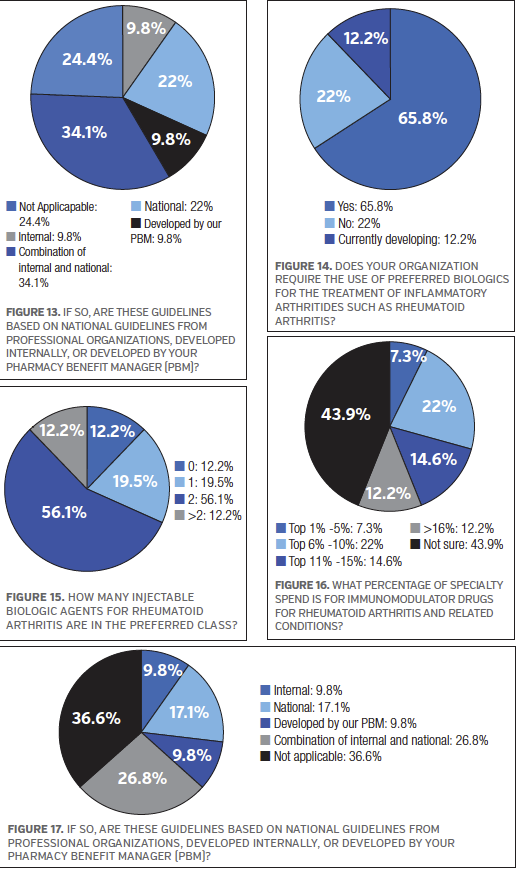
A large majority of the survey respondents (70.7%) said their organization currently has treatment guidelines in place for the treatment of RA. In terms of the guidelines they have in place, 34.1% of the survey participants said these guidelines are based on a combination of internally- and national-developed guidelines, while 22.0% said they are based on national guidelines from professional organizations and 9.8% said the guidelines are developed by their pharmacy benefit manager. See Figure 13.
When asked if their organization currently has prior authorization screening criteria for access to specialty drugs to treat RA, 73.2% of the survey participants said “yes.”
Because compliance with medications is an important component of RA treatment,3 the survey participants were asked if their organization has programs in place to improve compliance for RA medications; 61.0% answered “yes.”
A variety of drug therapies are used in the treatment of RA. Some medicines are used mainly to ease RA symptoms (eg, nonsteroidal anti-inflammatory drugs) and others are used to slow or halt the disease course and to inhibit structural damage (eg, corticosteroids, disease-modifying antirheumatic drugs [DMARDs], biologics, and Janus kinase [JAK] inhibitors).
To get a better understanding of treatment decisions based on RA drug therapies, the survey respondents were asked about drug classes for this disease state. The majority of the survey participants (80.5%) said their organization requires nonbiologic DMARDs to be used before a patient can be approved for a biologic. When asked if their organization requires prior authorization for any injectable biologic agents used in the treatment of RA (ie, specialty pharmacy drugs including the JAK inhibitors that are administered orally), 78.0% of the survey respondents answered “yes”. When asked if their organization requires the use of preferred biologics for the treatment of inflammatory arthritides such as RA, 65.9% of the survey participants said “yes,” while 22.0% said “no,” and 12.2% said “currently developing.” See Figure 14. More than half of the survey participants (56.1%) said 2 injectable biologic agents for RA are in the preferred class. See Figure 15.
When asked what percentage of specialty spend is for immunodulator drugs for RA and related conditions, 20% of the survey participants said the top 6% to 10%, while 14.6% said top 11% to 15%, and 12.2% said >16%. See Figure 16.
Psoriatic Arthritis
It has been estimated that up to 30% of people with psoriasis develop psoriatic arthritis, an inflammatory form of arthritis.4 The most common symptoms of psoriatic arthritis include fatigue, tenderness, pain, and swelling over tendons, swollen fingers or toes, stiffness or pain in joints, nail changes, and redness and pain of the eye. Psoriatic arthritis usually develops between ages 30 and 50, but can develop at any time including childhood.4 Similar to psoriasis, psoriatic arthritis varies from mild to severe depending on the number of joints that are affected. Psoriatic arthritis can manifest as spondylitis, enthesitis, and dactylitis. Genes, the immune system, and environmental factors are thought to play a role in the onset of the disease. If left untreated, individuals with psoriatic arthritis can have persistent inflammation, progressive joint damage, severe physical limitations, and increased mortality.
Like psoriasis, there is no known cure for psoriatic arthritis; it is often a lifelong disease that can flare and clear unpredictably. Treatments for psoriatic arthritis include nonsteroidal anti-inflammatory drugs, DMARDs, biologics, and new oral treatments.
Survey Results
More than half of the survey respondents (58.5%) said their organization currently has treatment guidelines in place for the treatment of psoriatic arthritis. For those survey participants who have treatment guidelines in place, 26.8% said they are based on a combination of internally- and national-developed guidelines, while 17.1% said they are based on national guidelines from professional organizations. See Figure 17.
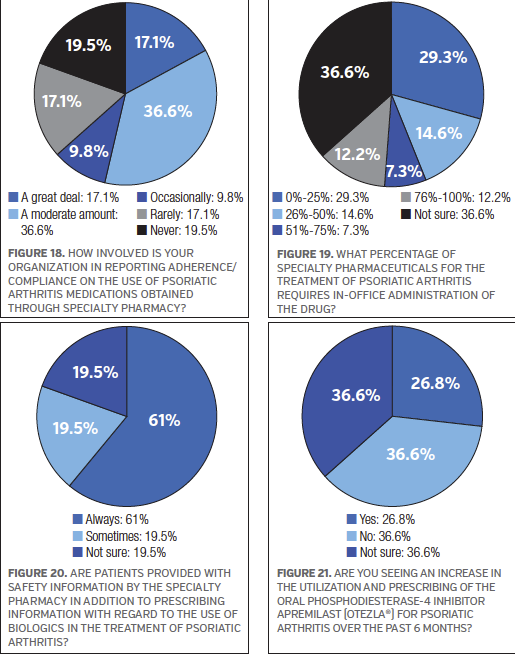
When asked if their organization currently has prior authorization screening criteria for access to specialty pharmacy drugs to treat psoriatic arthritis, 68.3% of the survey participants said “yes.” On the topic of adherence/compliance, 36.6% of the survey participants said “a moderate amount” with regards to how involved their organization is in reporting adherence/compliance on the use of psoriatic arthritis medications obtained through specialty pharmacy. Another 17.1% each said “a great deal” or “rarely.” See Figure 18.
Because some specialty pharmaceuticals used in the treatment of psoriatic arthritis require in-office administration, the survey participants were asked to select a percentage range that best represents their organization, and 29.3% of the respondents said 0% to 25%. Another 14.6% of the survey respondents said 26% to 50%. However, 36.6% of those who took the survey said they were not sure what percentage of specialty pharmaceutics for this disease state requires in-office administration of the drug. See Figure 19.
The majority of the survey participants (61.1%) said patients are “always” provided with safety information by the specialty pharmacy in addition to prescribing information with regard to the use of biologics in the treatment of psoriatic arthritis. See Figure 20.
Currently, there is no known cure for psoriatic arthritis. Apremilast (Otezla) is one example of a new oral treatment for psoriatic arthritis. It is a phosphodiesterase-4 inhibitor that received FDA approval5 in 2014. The survey participants were asked if they are seeing an increase in the utilization and prescribing of apremilast for psoriatic arthritis over the past 6 months, and 36.6% said “no” compared with 26.8% who said “yes.” See Figure 21.
Conclusion
Individuals with RA and psoriatic arthritis often require lifelong management and support. Although the majority of survey respondents said their organizations have treatment guidelines in place, there is room for improvement considering 29.3% and 41.5% of the survey respondents reported their organization does not have treatment guidelines in place to treat RA and psoriatic arthritis, respectively.
Compliance and adherence measures are also vital components in managing and treating RA and psoriatic arthritis. Yet, 39.0% of the survey respondents said their organization lacks programs to improve compliance for RA medications. For psoriatic arthritis, 36.6% classified their organization’s involvement in reporting adherence/compliance on medications obtained from a specialty pharmacy as “a moderate amount” and 19.5% reported that their organization is “never” involved.
Prior authorization screening criteria for access to specialty drugs is another important issue in the managed care arena. The majority of the survey participants indicated that their organization requires this step for treating RA (73.2%) and psoriatic arthritis (68.3%).
The survey respondents also shared their general thoughts and concerns regarding these disease states through an open-ended section of the survey (Box). Whereas medication adherence has always been a challenge in managing RA and psoriatic arthritis, convincing patients to follow formulary protocol adds to this struggle. Direct-to-consumer advertising and strong marketing campaigns for biologics have many patients clamor for newer and more expensive treatments rather than trying older, more cost effective treatment plans. As stated by one pharmacy director, education will be key to achieving success in treatment.
REFERENCES
1. The American Autoimmune Related Diseases Association. The Cost Burden of Autoimmune Disease: The Latest Front in the War on Healthcare Spending. https://www.diabetesed.net/page/_files/autoimmune-diseases.pdf. Published 2011. Accessed August 15, 2016.
2. What is rheumatoid arthritis? Arthritis Foundation website. https://www.arthritis.org/about-arthritis/types/rheumatoid-arthritis/what-is-rheumatoid-arthritis.php. Accessed August 15, 2016.
3. van den Bemt BJ, Zwikker HE, van den Ende CH. Medication adherence in patients with rheumatoid arthritis: a critical appraisal of the existing literature. Expert Rev Clin Immunol. 2012;8(4):337-351.
4. About psoriatic arthritis. National Psoriasis Foundation website. https://www.psoriasis.org/psoriatic-arthritis. Accessed August 15, 2016.
5. FDA approves Otezla to treat psoriatic arthritis. FDA website. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm390091.htm. Updated March 24, 2014. Accessed August 15, 2016.




















